Chapter 3 + some chapter 14 - Microbial Metabolism & Metabolic Diversity of Microbes
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
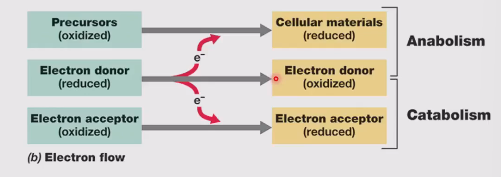
Catabolism
Reactants → products
Creates ATP from ADP (Energy can be used for anabolism or for movement, transport of nutrients)
Exergonic reactions: reactions that enable ATP-synthesis (energy is released)
A reaction that gives off energy has a negative delta G
Anabolism
Precursors → cellular materials
Uses ATP
Exergonic reactions
Reactions with a negative delta G (spontaneous)
These enable ATP-synthesis
ATP is subsequently used for synthesis of cell components
Reducing power
Is essential for:
Energy generation (catabolism)
Biosynthesis of cell components (anabolism)
Energy sources for microbes
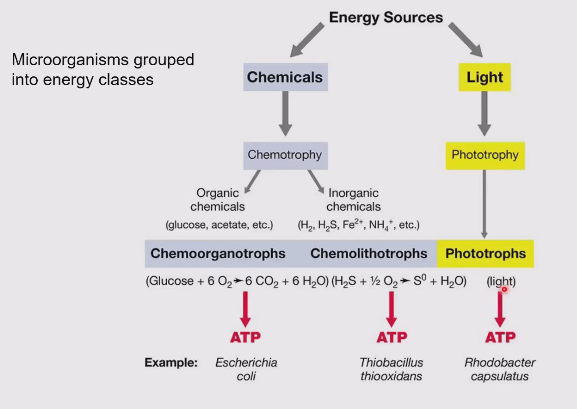
Metabolic classes of microbes

Reduction potential
Tendency to donate electrons (so potential to reduce others because is oxidized itself)
Reduced substance of a redox couple with a more negative E0 donates electrons to the oxidized substance of a redox couple with a more positive E
What happens when the gap becomes larger in the redox tower?
More negative which also releases more energy
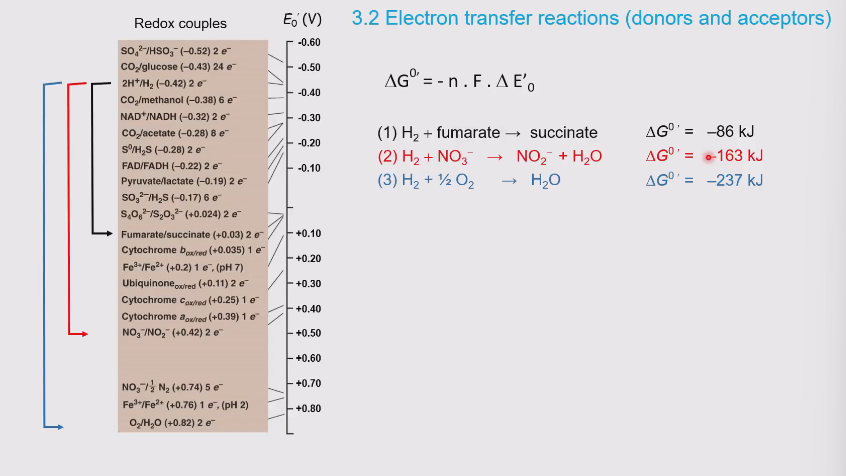
Redox tower
Represents the range of possible reduction potentials
Substances toward the top (so all the negative values) prefer to donate electrons
Substances toward the bottom (oxidized, so all the positive values) prefer to accept electrons
The farther the electrons “drop”, the greater the amount of energy released
Reduction potential
Potential of reducing others (being oxidized itself)
The tendency to donate electrons (V)
More negative is better electron donors
More positive is better electron acceptors
In redox tower, the electron acceptors are at bottom and donors at the top.
Molecules that are more neutral (e.g. -0.320) usually bridge the gap between donating and accepting. A good example is NAD/NADH
Electron carriers
Electrons are never free in the cell, but always bound to a carrier
Electrons from energy source → electron carrier → terminal electron acceptor (e.g. O2)
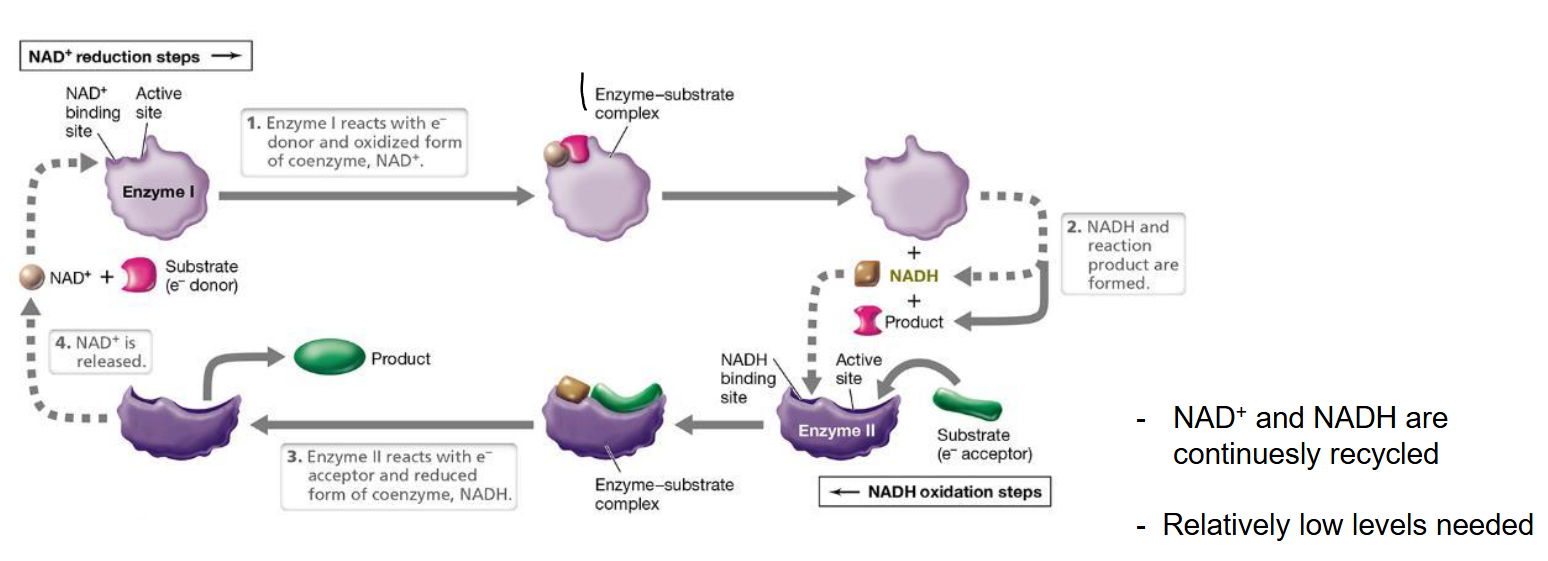
Cells contain only a limited amount of electron carriers that means that the reduced form must be continuously oxidized (NADH → NAD+), therefore relatively low levels of NAD are needed
Most common NAD+ → NADH
What does the value of G tell us?
If the reaction is exergonic or endergonic
Exergonic: Can be a potential energy source for the cell
Endergonic: Requires an energy input to proceed
The larger the gap between the half reactions in the redox tower, the more energy and the larger G.
The process of recycling NAD as electron carrier
Method to recycle NAD+/NADH
Enzyme I accepts NAD+ and substrate (e- donor)
This releases NADH and the rest of the substrate without the electron
Now the NADH binds to enzyme II with the substrate (e- acceptor)
Afterwards the product has the e- and NADH is turned back into NAD+. The cycle begins again.
What molecules can store this high amount of energy coming from electrons in the short-run
ATP (most important, for short term processes)
Pyruvate
Acetyl-CoA
Acetyl phosphate
Glucose 6-phosphate
Lots of these contain phosphate → this is because the carbon phosphate group contains a lot of energy
Long term energy storage
Involves biosynthesis of insoluble polymers that can be oxidized to generate ATP
Examples in prokaryotes:
Glycogen
Poly-B-hydroxybutyrate
Polyphosphate
Elemental sulfur (S)
Essences of catabolism
Substrate-level phosphorylation → ATP synthesis DIRECTLY coupled to an energy generating (exergonic) reaction (Basically ADP → ATP)
Electron transport phosphorylation (=oxidative phosphorylation) → ATP synthesis coupled to electron transport, via a respiratory chain. Redox reactions enable ATP synthesis INDIRECTLY
Photophosphorylation> light energy used for making ATP
Fermentation
Anaerobic catabolism in which organic compounds donate and accept electrons
no electron acceptor is available - therefore oxidized intermediates used as electron acceptor
Only substrate level phosphorylation
2 ATP produced → rest of the energy is still present in the products (lactate, ethanol)
Respiration
A metabolic process in which an electron donor is oxidized, transferring electrons through an electron transport chain to a terminal electron acceptor, which may be oxygen (aerobic respiration) or another compound (anaerobic respiration), resulting in ATP production.
Steps of fermentation
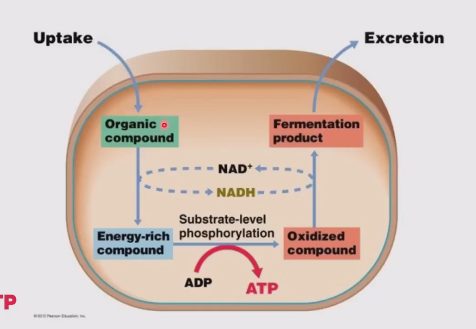
An organic compound (e.g. glucose) is taken up by the cell
The cell oxidizes this compound and forms and energy-rich compound. (To do this NAD+ becomes NADH)
This energy-rich compound is used for substrate level phosphorylation where ADP is turned into ATP and that forms an oxidized compound
The cell then reduces the compound again and the fermentation product is excreted (also NADH → NAD+)
Glycolysis
Investment stage: Start with glucose where two ATP are invested
Glucose is separated into two (C3) molecules which each have two phosphate groups (= lots of energy!!)
Pay-off stage:
NAD+ is turned into NADH
The C3 molecules are dephosphorylated to form ATP
Pyruvate is formed
For fermentation:
Since we now have NADH which cannot accept any electrons, the pyruvate will receive the electrons
The pyruvate can turn into lactate (muscles or certain bacteria) or 2 ethanol and 2 CO2 products (yeasts)
In total two ATP are invested and four ATP are received back.
What do yeast produce in glycolysis?
2 ethanol
2 CO2
What do lactic acid bacteria or your muscles produce?
2 Lactate
Energetic and redox considerations for fermentative microbes
Dependent on substrate level phosphorylation → little energy
Shortage on external electron acceptors; difficult to reach redox balance
This holds especially for strict fermentative microbes.
Besides reduction of intermediates for redox balance, how else can redox be balanced?
By production of H2
Lactic acid bacteria
Gram +
Not sporulating
Oxygen tolerant (indifferent)
Lactic acid as main end product
What are the two types of lactic acid bacteria?
homofermentative: make only lactic acid
Heterofermentative: Make lactic acid but also ethanol and CO2
Heterofermentative will do other pathways besides glycolysis
How is ATP generated in respiration?
By oxidative phosphorylation
Drive by the “proton motive force”, which is build up over the cytoplasmic membrane of prokaryotes.
Citric acid cycle
pathway through which pyruvate is completely oxidized
the electrons that are released are transferred to NAD+ and FAD
three intermediates are also precursors for anabolic reactions
Start with C6 molecule
Then down to C5, to C4
C4 combines with C2 to make C6, process starts again.
The electron transport chain
Complex I - NADH turns into NAD+ and electrons enter the cycle. Protons are pumped across the membrane
Complex II - FADH2 turns into FAD
Complex III - Quinones (made in I & II) go into complex III, flow through the cytochromes and eventually through complex IV to the oxygen. Also pumps protons across the membrane
Complex IV - the electrons from complex III that have gone through the cytochrome go to the oxygen and the complex pumps out two protons.
pH in electron transport chain
Protons are pumped out and therefore the outside becomes electrically positive and acidic
The inside becomes electrically negative and alkaline
What is the proton motive force used for?
ATP production by ATP synthase
Active transport
Movement
Electron transport chain in anaerobic respiration
Is possible, instead nitrate is reduced but this will give far less ATP
Order of type of respiration
If oxygen is available → it will respire with oxygen
If oxygen runs out, it will try to switch to nitrate respiration
If nitrate runs out, it will do fermentation because there is no longer an electron acceptor available
Respiration vs. Fermentation
Respiration | Fermentation | |
Products | 6H2O and 6CO2 | 2CO2 and 2 Ethanol |
ATP | 38 ATP | 2 ATP |
Gibbs free energy | -2844 kJ | -226 kJ |
Applications of anabolism
Making RNA/DNA
Making fatty acids
How much ATP does glycolysis and CAC produce? (aerobic respiration)
38 ATP
How much ATP does fermentation produce from glucose?
2 ATP per glucose
Flexibility of respiration in bacteria
Bacteria can often do both aerobic and anaerobic respiration
E.g. E. coli grows by aerobic respiration (so oxygen is electron acceptor), fermentation (no electron acceptor), or a simple form of anaerobic respiration (with nitrate)
What is the Embden-Meyerhof-Parnas pathway?
Another word for glycolysis
Which intermediate compound(s) in the citric acid cycle is/are often used for biosynthetic pathways as well as carbon catabolism?
Enzymes: α-ketoglutarate, oxaloacetate, and succinyl-CoA
In what form is nitrogen commonly found?
In inorganic forms
Three elements that all microbes need
Phosphorus
Selenium
Sulfur
What class of macromolecules in microbes contributes the most to biomass?
Proteins