Glucose Transport and Glycogen
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
What are the two types of glucose transporters?
•GLUcose Transporters (GLUT)
•Sodium-Glucose Linked Transporters (SGLT)
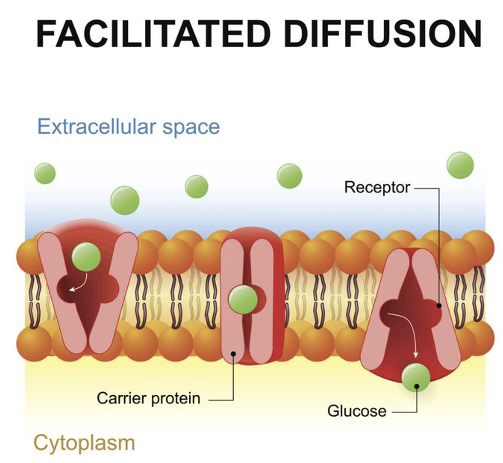
GLUT Transporters*
Operate by facilitated diffusion
Glucose binds to receptor of membrane protein
Conformation change releases glucose into cytoplasm
Does not consume energy
GLUT1*
•Expressed in all cell types
•Basal glucose uptake
•Most highly expressed in erythrocytes, brain, and placenta
•Glucose transport is not insulin-dependent
•Membrane expression increases with reduced blood glucose and decreases with increased glucose levels
GLUT2*
•Bidirectional glucose transport
•Found in hepatocytes, pancreatic β-cells, renal and small intestinal epithelial cells
•Glucose, galactose, and fructose are transported from intestine to portal circulation
•Not insulin-dependent
GLUT3*
•Non insulin-dependent transporter
•Neurons (neuronal GLUT)
•Also found in the embryo, sperm, leukocytes, and some cancer cells
•Higher glucose affinity and transport capacity compared to GLUT1, 2, & 4
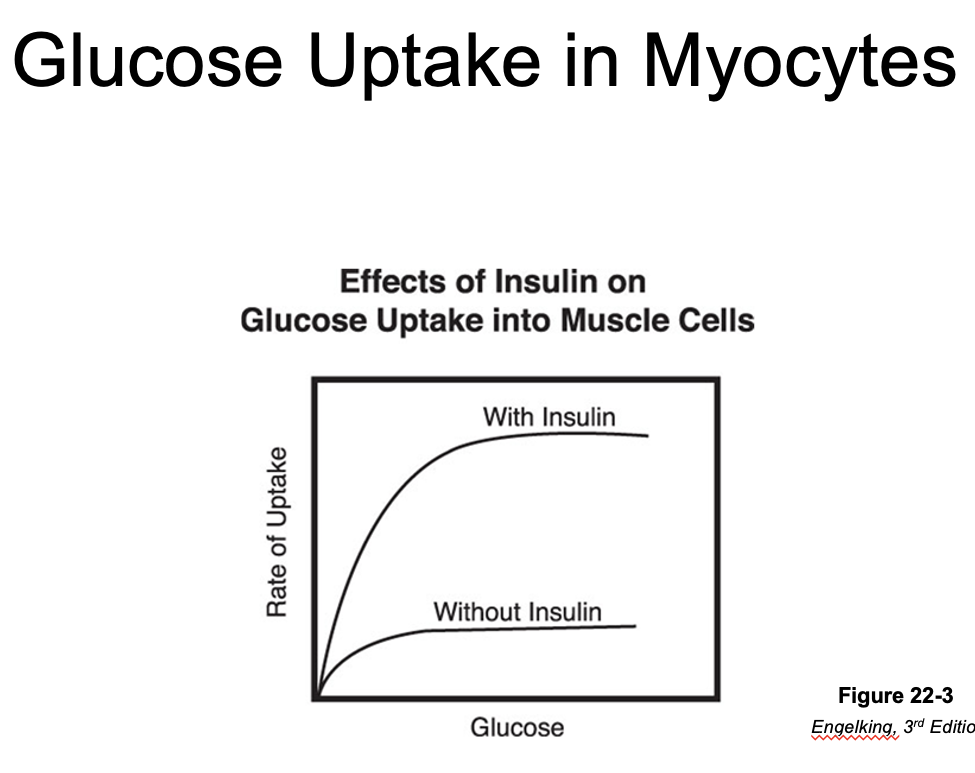
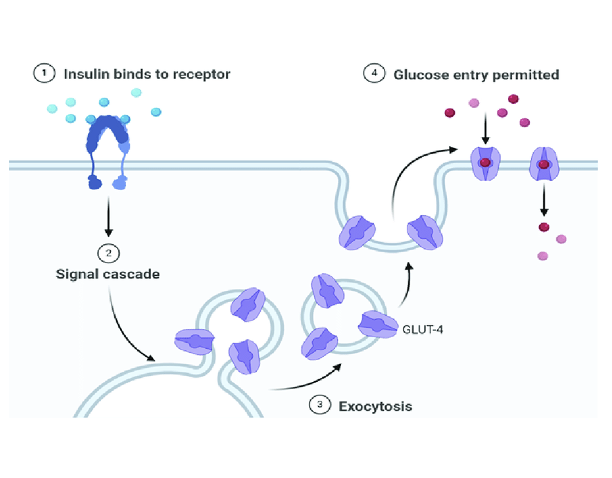
GLUT4*
•Key insulin-dependent transporter
•Found in adipocytes, skeletal and cardiac myocytes
•Insulin stimulates translocation of GLUT4 to the cell membrane
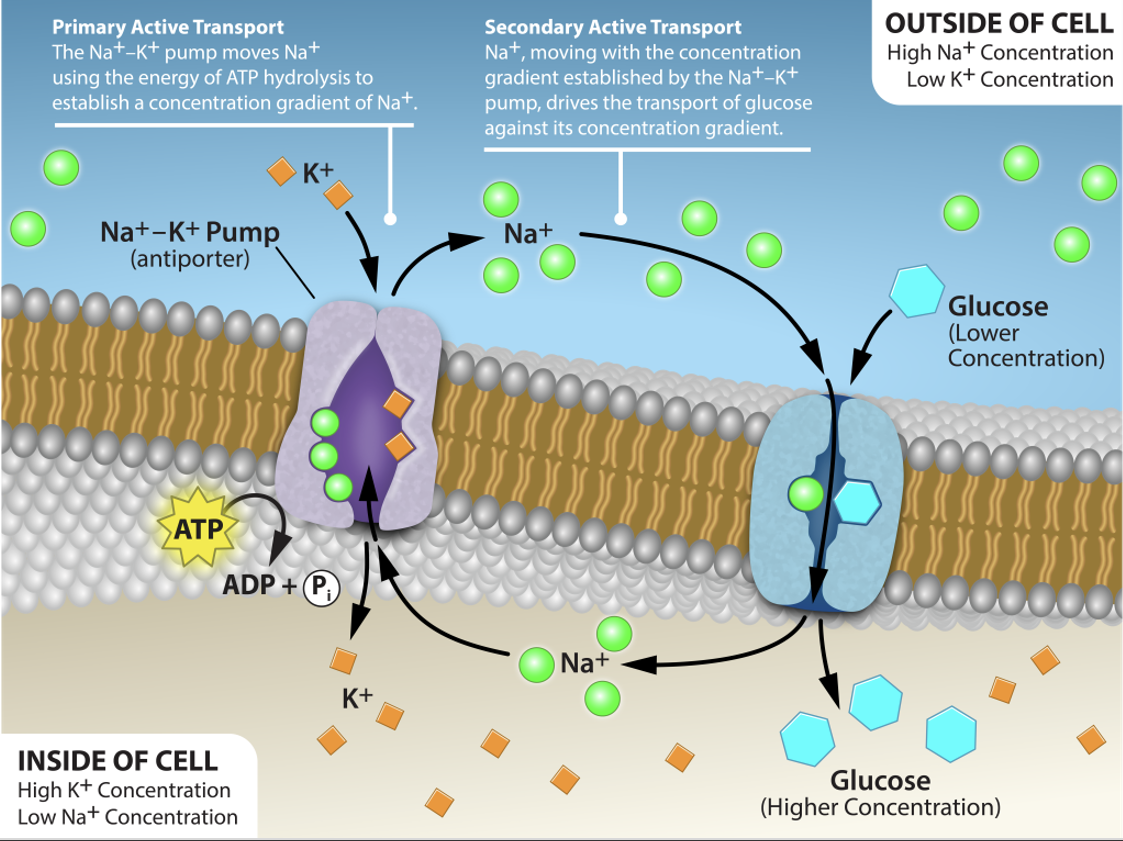
Sodium-Dependent Glucose Transporters*
SGLT1 and SGLT2
Insulin-independent transporters
Use Na+ gradient to drive transport
Intracellular Na+ is much lower than extracellular fluid Na+
Na+ gradient is maintained by Na/K ATPase pump
Na+ and glucose are transported together (symport)
Allows glucose to be moved against the concentration gradient
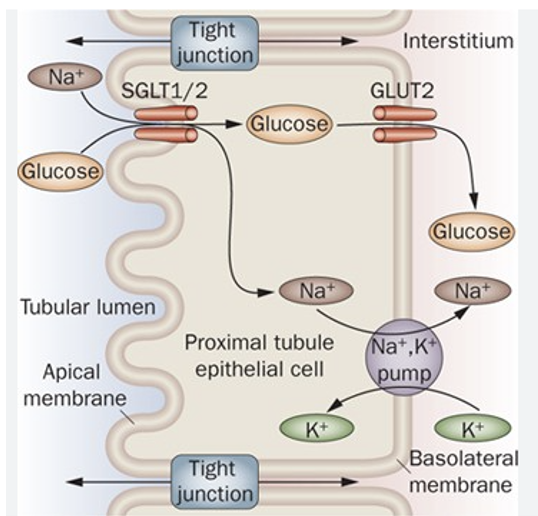
SGLT1 and SGLT2*
SGLT1 and SGLT2 are expressed in the renal tubular epithelium of the proximal tubule
SGLT2 is only present in the kidney
Resorb glucose from the glomerular filtrate
Under normal conditions, virtually all of the glucose is resorbed before urine leaves the kidney
At very high blood glucose levels, not all glucose can be resorbed, and glucosuria occurs
SGLT2 inhibitors (e.g., bexagliflozin) have recently been used therapeutically to decrease blood glucose levels in cats (and humans) with diabetes by “spilling” glucose in the urine
Competitive inhibitor of glucose binding site of SGLT2
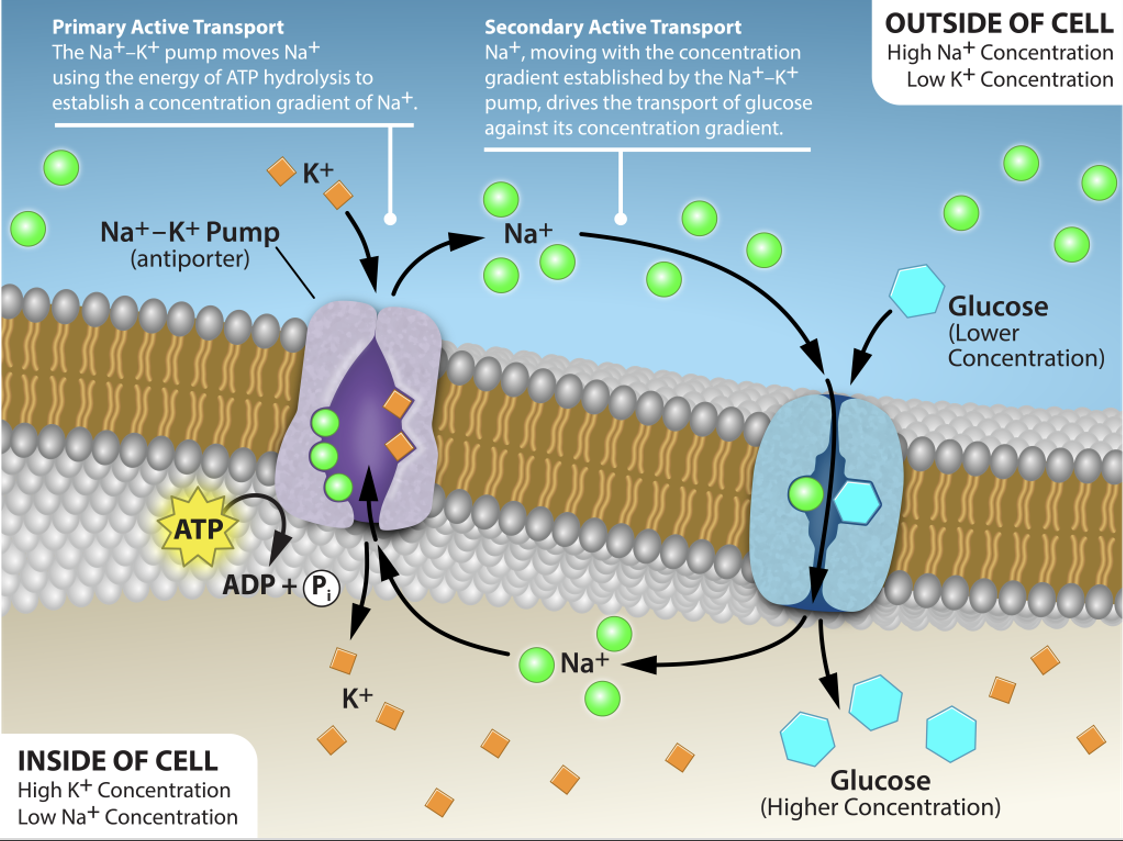
Why doesn’t glucose diffuse back out of the cells?
GLUT2 is bidirectional
Diffusion would cause glucose to move from areas with higher concentration to areas with lower concentrations
Glucose “Trapping”
Phosphorylation to glucose-6-phosphate (G-6-P)
Enzymes hexokinase and glucokinase phosphorylate glucose (converting ATP to ADP)
G-6-P is not transported by GLUT2 transporter
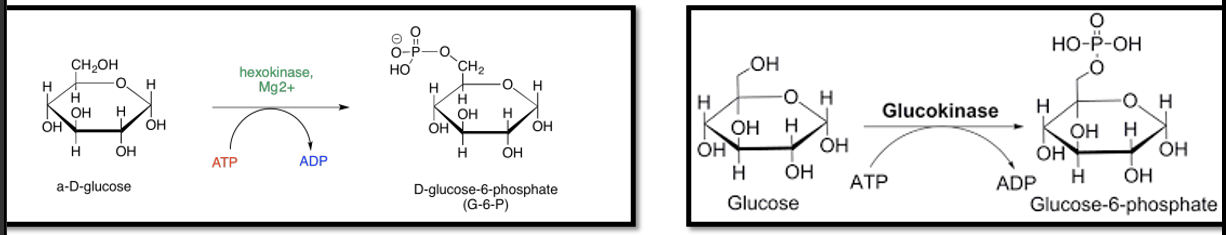
Glucose Phosphorylation
•Phosphorylated glucose can be used for synthesis of glycogen
•Also the first step for glycolysis
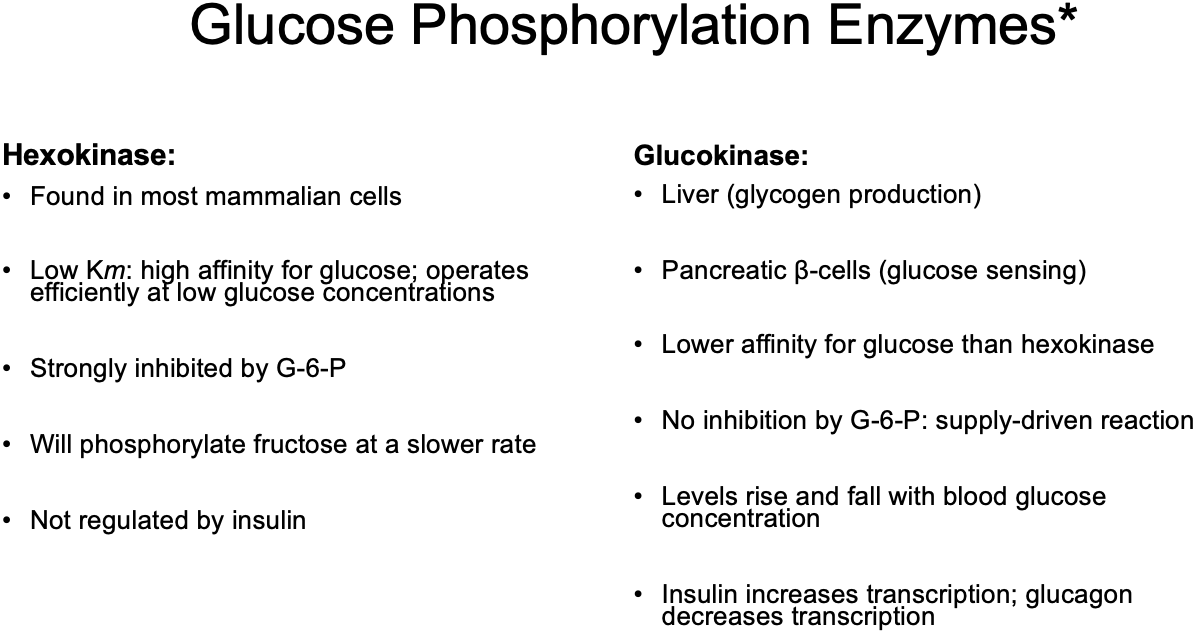
Hexokinase
a type of Glucose Phosphorylation Enzyme:
Found in most mammalian cells
Low Km: high affinity for glucose; operates efficiently at low glucose concentrations
Strongly inhibited by G-6-P (its own product)
Will phosphorylate fructose at a slower rate
Not regulated by insulin
Glucokinase
a type of Glucose Phosphorylation Enzyme:
Liver (glycogen production)
Pancreatic β-cells (glucose sensing)
Lower affinity (weaker attraction; higher concentrations of glucose needed to operate efficiently) for glucose than hexokinase
No inhibition by G-6-P: supply-driven reaction (the more glucose, the more phosphorylation)
Levels rise and fall with blood glucose concentration
Insulin increases transcription; glucagon decreases transcription
Glucose Phosphorylation Enzymes*
Hexokinase
Glucokinase
Glucokinase: Species Variations*
Hepatic glucokinase is needed to respond to elevated levels of blood glucose (high dietary intake)
Species that take in low amounts of glucose from their natural diets (starch goes to microbes before getting to abomasum) lack hepatic glucokinase:
Ruminants (generate short-chain fatty acids from plant material)
Strict carnivores (high protein, low carbohydrate diet)
These species do have pancreatic glucokinase (glucose sensing)
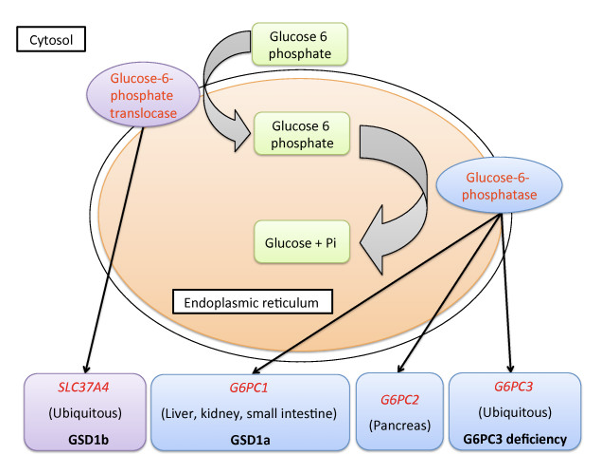
Glucose-6-Phosphatase (G6Pase)
Enzyme that removes phosphate from G-6-P*
Found in hepatocytes and intestinal & renal cells*
Expression is suppressed by insulin (wants to trap glucose in cells)*
Allows glucose to be released from the hepatocytes via the GLUT2 transporter*
Muscle cells and adipocytes lack G6Pase (do not export glucose)*
Glycogen
Storage form of carbohydrates/glucose
Polymer of glucose
Each glycogen molecule can contain up to 30,000 glucose residues
Hydrophilic, hydrated (65% water)
Present in cytosol (myocytes and hepatocytes contain most of the body’s glycogen)
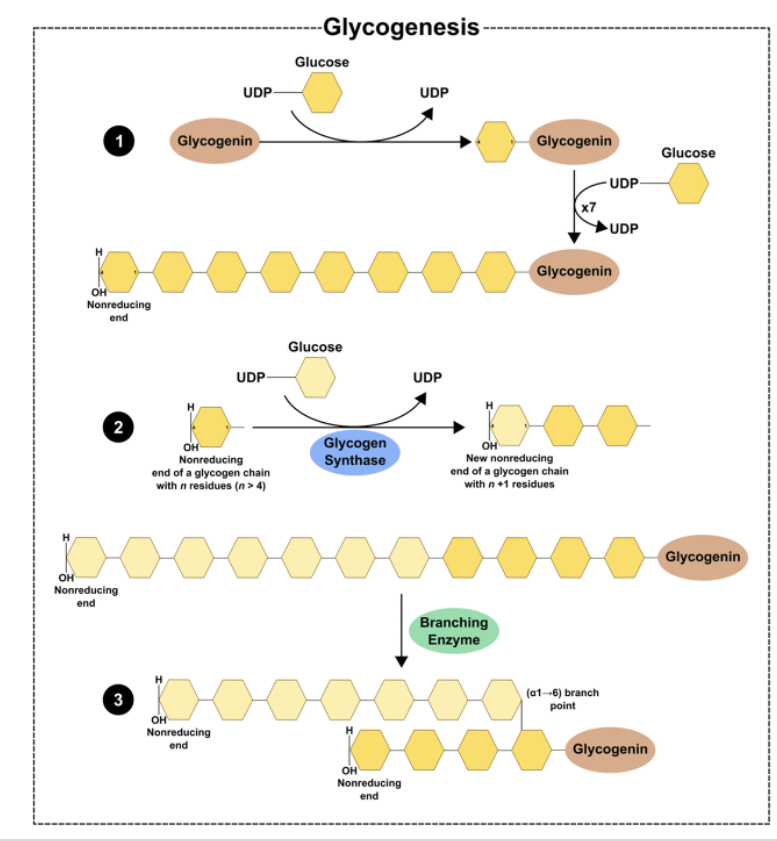
Glycogenesis
formation of glycogen
Glycogenolysis
breakdown of glycogen
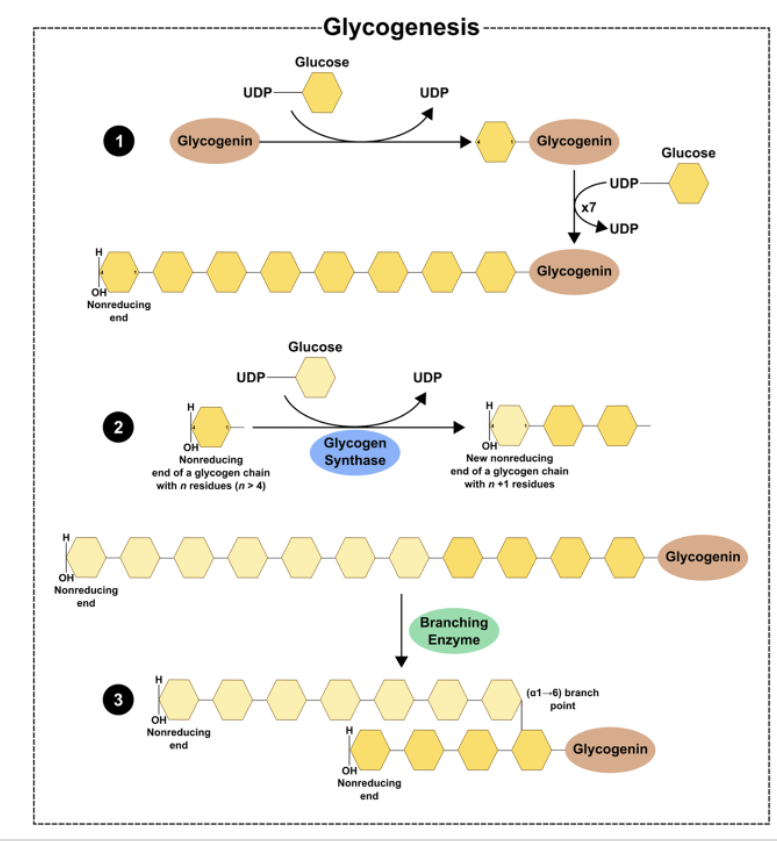
Glycogen Synthesis: First Steps
Phosphorylation of glucose to G-6-P via the enzymes:
Hexokinase
Glucokinase
Conversion of G-6-P to G-1-P
Enzyme: Phosphoglucomutase
Cofactor: Mg2+
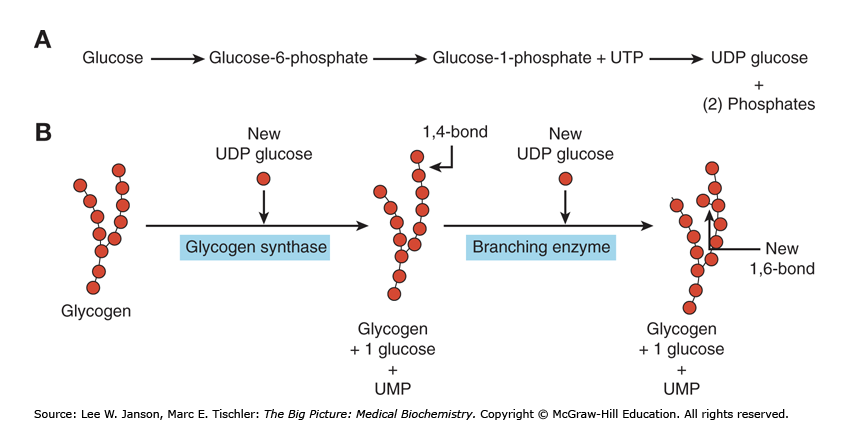
Glycogen Synthesis: Synthesis of UDP-Glucose
G-1-P is then converted to uridine diphosphate-glucose (UDP-Glc)
Enzyme: UDP-Glc pyrophosphorylase
Catalyzes the formation of UDP-glucose from glucose-1-phosphate and UTP.
(G-1-P + UTP → UDP-glc)
Possible Fates of UDP-Glucose
Glycogen synthesis
Uronic acid pathway
Lactose synthesis (mammary gland)
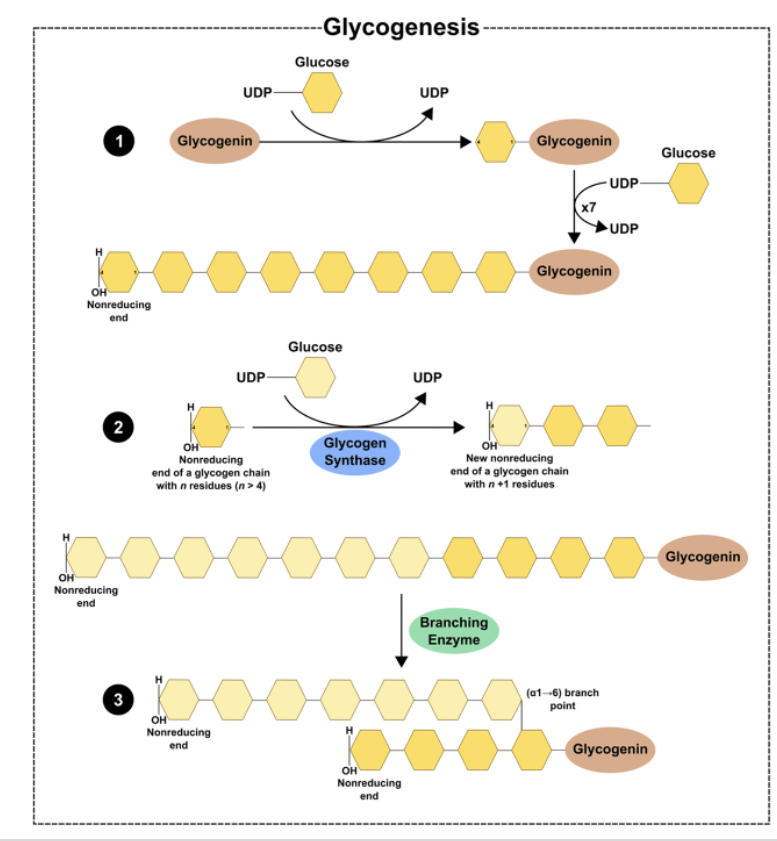
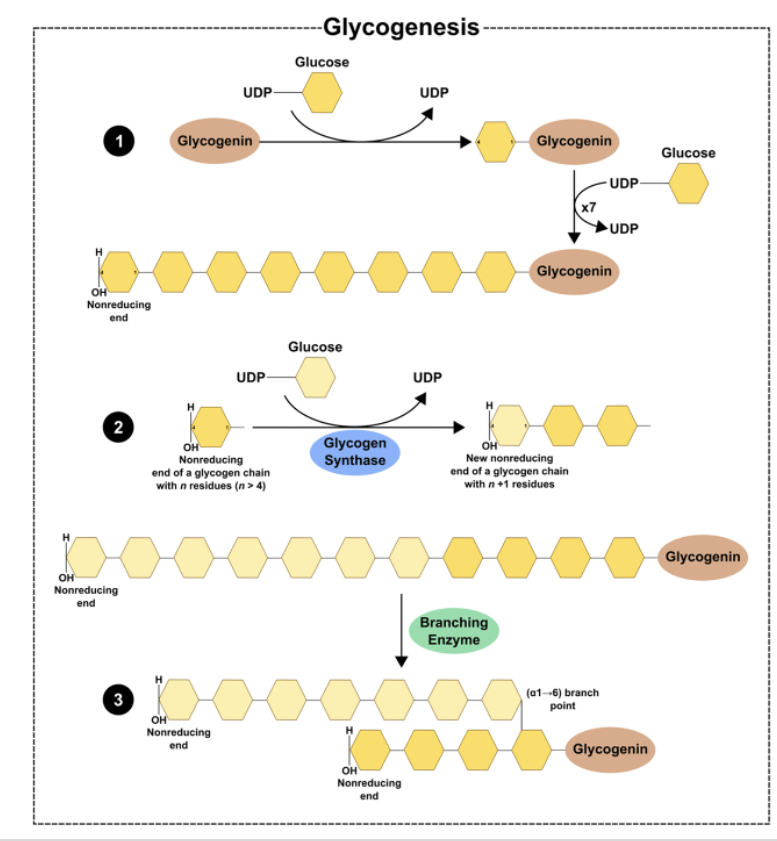
Glycogen Synthesis: Glycogenin*
•Glycogenin is an enzyme that catalyzes the polymerization of the first few glucose molecules forming an oligosaccharide (3-15)
•Protein forms the core of the glycogen complex
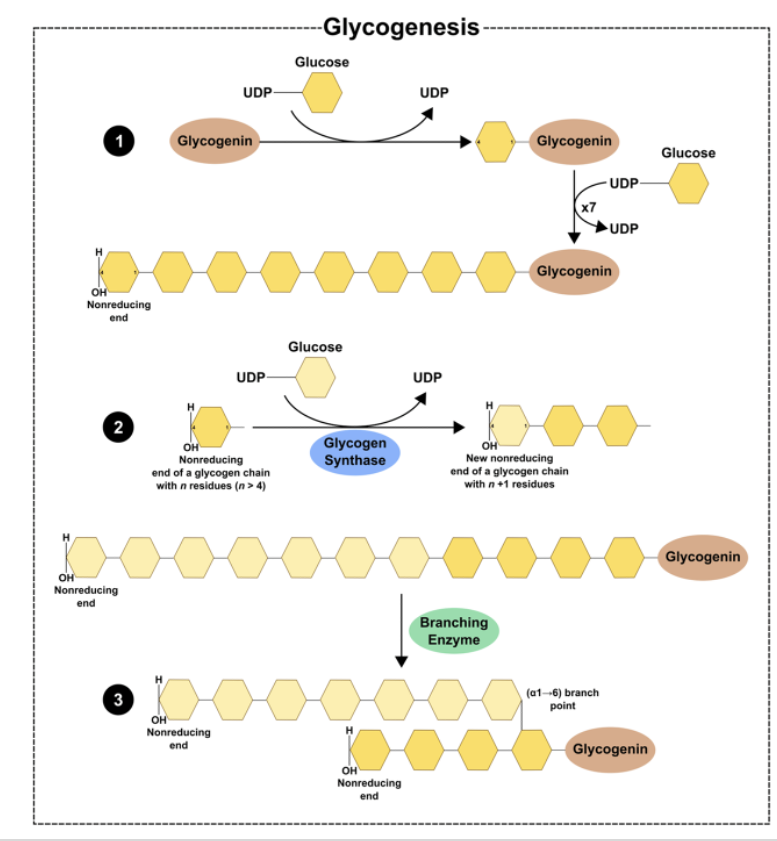
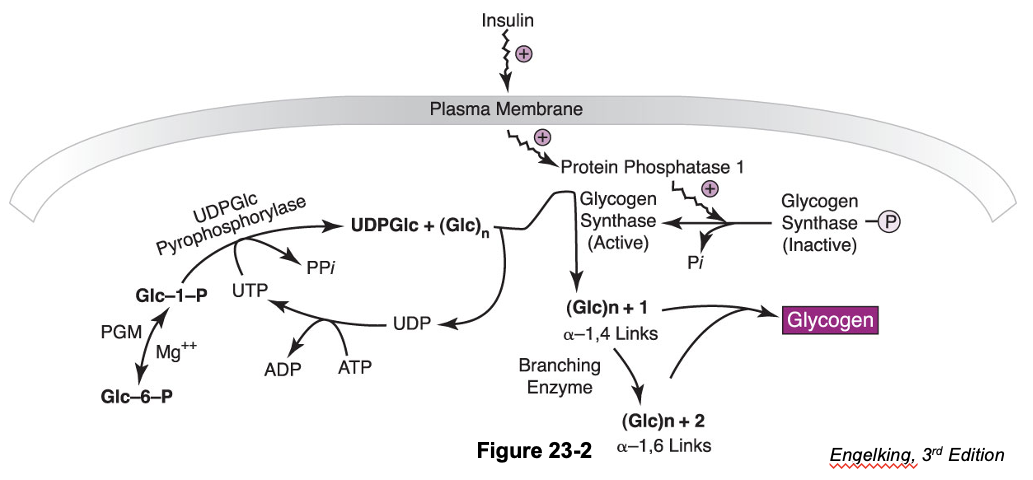
Glycogen Synthesis: Elongation and Branching*
Glycogen synthase:
Enzyme that catalyzes the elongation of a chain by addition of glucose molecules in a linear fashion
- Catalyzes the transfer of the glucosyl residue of UDP-Glc onto glycogen via α-1,4 glycosidic bonds
Rate-limiting step of glycogenesis
Activated by dephosphorylation (protein phosphatase 1)
Branching enzyme transfers glucose chains
Block of 6-7 units transferred to another chain
Regulation of Glycogen Synthesis
In the fed state (of monogastrics) glucose levels are high, and insulin is secreted
Insulin stimulates glucose transport, utilization, and storage as glycogen
Glycogenolysis
Mobilization of glucose from glycogen stores
Not the reverse of glycogenesis: separate pathway
Glycogen phosphorylase
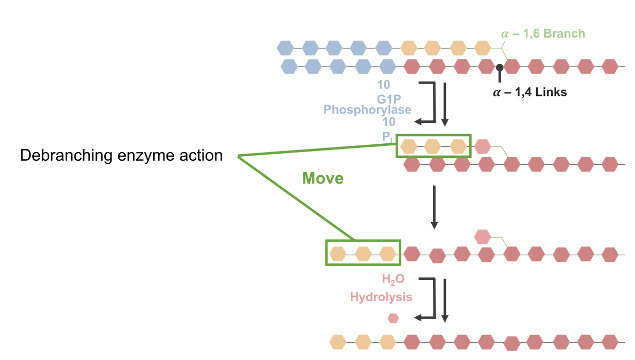
Releases G-1-P (90%)
Shortens chains to within 4 glucose molecules from branch
Will not break down chains after they reach 4 residues in length
Debranching enzyme
Disassembles branch points
Transfers to elongate chain (then elongated chain is broken down by glycogen phosphorylase)
Glycogen Phosphorylase
Catalyzes rate-limiting step of glycogenolysis*
Pyridoxal phosphate as coenzyme
Cleavage of 1,4 linkages of glycogen to yield G-1-P*
Muscle form is distinct from liver form (isozymes)
Glycogenolysis: Products*
G-1-P → G-6-P via phosphoglucomutase (reversible reaction)
Liver
Can convert G-6-P into glucose by glucose-6-phosphatase
- Export from hepatocytes for blood glucose
G-6-P → glycolysis for energy production
Muscle
G-6-P used for glycolysis
Regulation of Glycogenolysis: Glycogen Phosphorylase
Glycogen phosphorylase activation:
Activated by phosphorylation
- Inhibited by ATP, G-6-P, glucose (liver)
Inactivated by protein phosphatase-1 (dephosphorylation)
- Protein phosphatase-1 Activated by insulin (wants to keep glucose in cell)
Glycogen phosphorylase is stimulated by hormones/neurotransmitters:
Glucagon
Epinephrine
Norepinephrine
Cortisol
Glycogenolysis Regulation: Glucagon and Glycogen Phosphorylase
Glucagon (hormone) binds cell membrane receptor
Adenylate cyclase is activated, cAMP is increased
Protein kinase A is activated, which then activates phosphorylase kinase
Phosphorylase kinase
Activates glycogen phosphorylase → glycogenolysis
Inhibits glycogen synthase
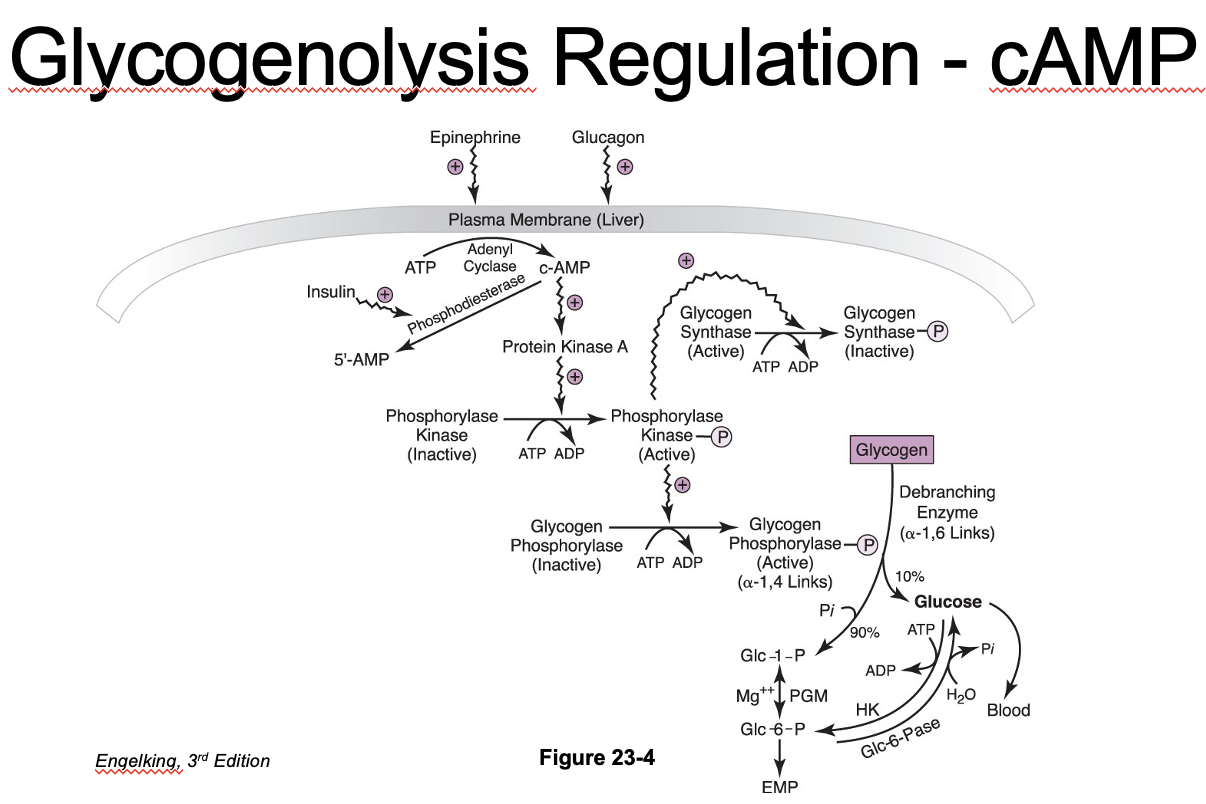
Glycogenolysis Regulation: Ca2+ Messaging
Phosphorylase kinase can also be activated by a calcium second messenger pathway in muscle (and liver)
Independent of cAMP
Calcium can be increased via nerve impulses, hormones or muscle contraction
Synchronizes glycogenolysis with muscle contraction
Additive effect with cAMP
Glycogen Storage Diseases
Inherited disorders of glycogen metabolism caused by deficient or defective enzymes
Affected animals have hypoglycemia and glycogen accumulation in tissues
Defective G6Pase: glycogen can’t be broken down properly
Defective branching enzymes: abnormal long chains with low solubility
Glycogen precipitates in cells and causes cell injury
Generally rare diseases in practice
Signs become apparent soon after birth (may die in utero)
Failure to grow/thrive, anorexia, weakness, abdominal distension, vomiting
Fasting hypoglycemia
Abnormal glycogen accumulation in tissues (hepatocytes or myocytes)
Can be identified by genetic testing (carriers)
Supportive care or gene replacement therapy?
Glycogen Storage Diseases: Classification
What would be the consequences of these mutations?
•Type I: glucose-6-phosphatase mutation (Maltese dog, Border collie)
•Type II: glycogen debranching enzyme (Lapland terriers)
•Type III: glycogen debranching enzyme (German shepherd dog, Akita)
•Type IV: glycogen branching enzyme (Norwegian Forest Cats)
•Type VII: phosphofructokinase (English springer spaniel, American cocker spaniel, whippet, mixed breed)
Glycogen Storage Disease in Cats*
Type IV (glycogen branching enzyme defect) in Norwegian Forest cats•
Autosomal recessive genetic defect: Affected kittens may die soon after birth, but some can appear normal until ~5 months of age. Clinical signs are fever and muscle tremors, progressing to generalized muscle atrophy and death.
Glycogen Storage Diseases in Horses
AKA PolySaccharide Storage Myopathy or PSSM
Glycogen accumulates in muscle tissue, causes “tying up” syndrome and muscle tremors
PSSM1 is from a mutation of glycogen synthase 1 (GYS1) gene
Quarter horses, draft horses, Appaloosas, and other breeds
PSSM2 (unknown defect)
Arabian/Warmblood, Quarter horse
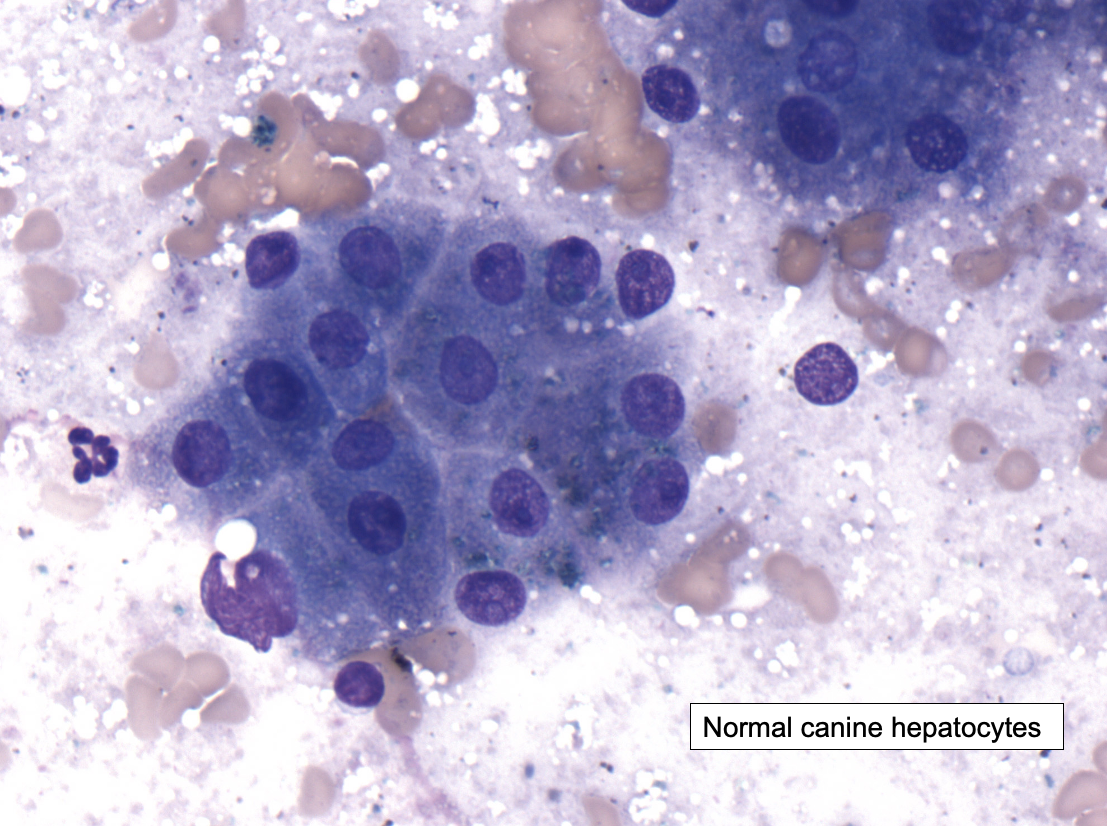
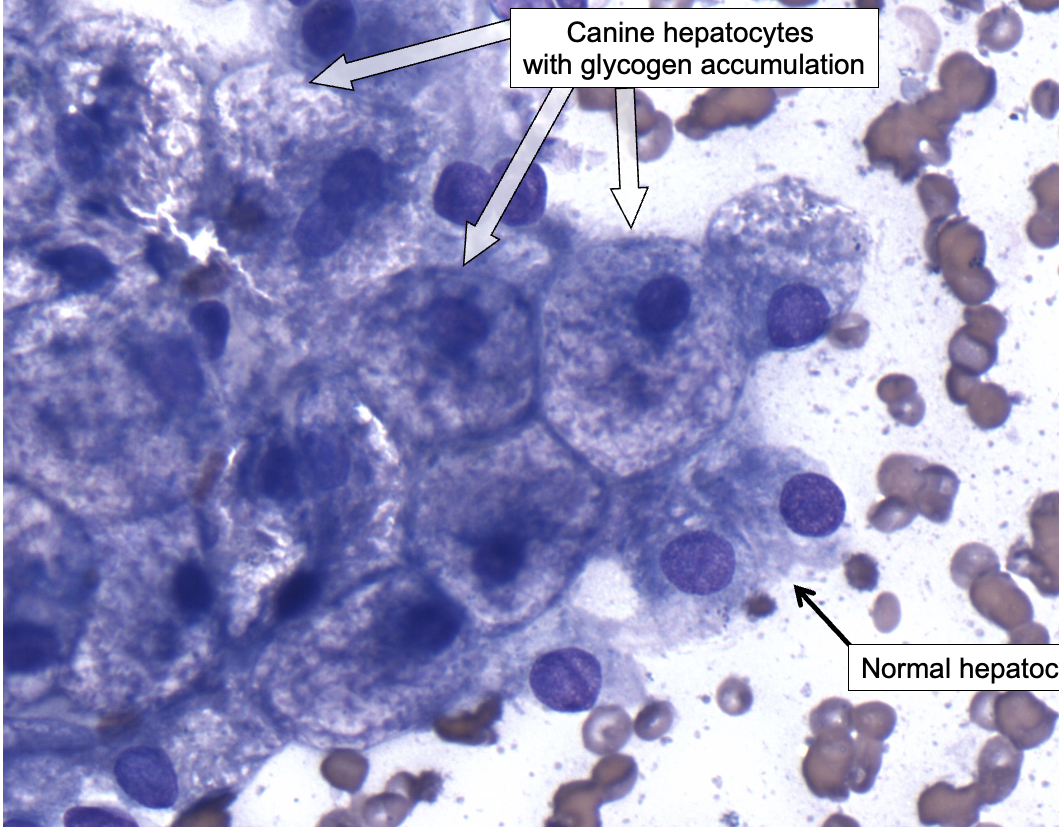
Clinical Relevance:
Vacuolar Change in Canine Hepatocytes
Increased glycogen deposition can cause swelling of hepatocytes, visible with light microscopy as diffuse vacuolar change.
“Hydropic change” can have a similar appearance
This can occur with glucocorticoid treatment (e.g., prednisone) or hyperadrenocorticism in dogs, which stimulates gluconeogenesis (leading to increased glycogen synthesis).
Glucocorticoids in general have an “anti-insulin” effect.
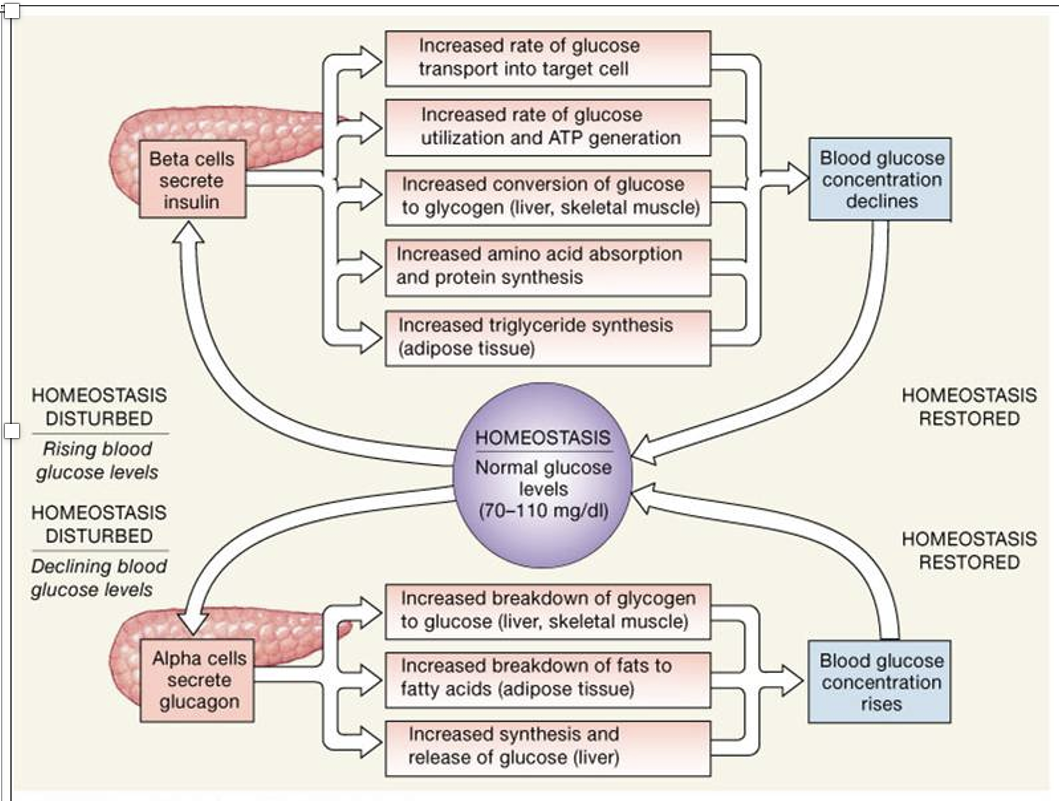
Blood glucose (“Glycemia”) is highly regulated
Glucagon (releases stored glucose) vs. Insulin (increases glucose storage)
Production/release vs. usage/consumption
Anabolic vs catabolic states
Intake vs. storage