Sigmatropic Rearrangments (I + II)
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
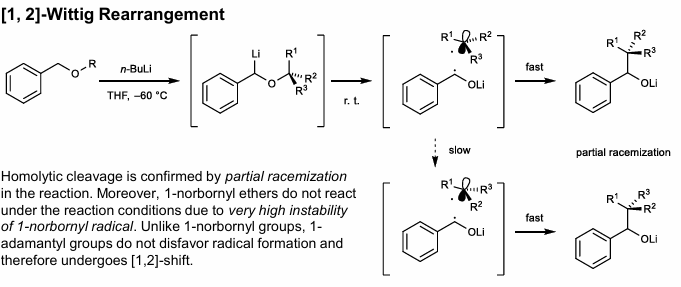
1,2-Wittig Rearrangement
Benzylic or propargylic stabilization required
Radical mechanism, not concerted.
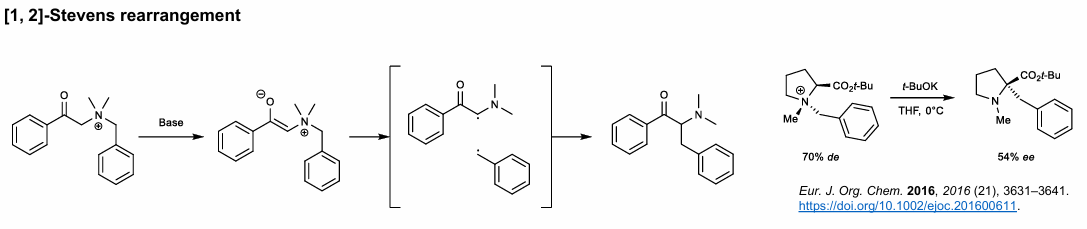
1,2-Stevens Rearrangement
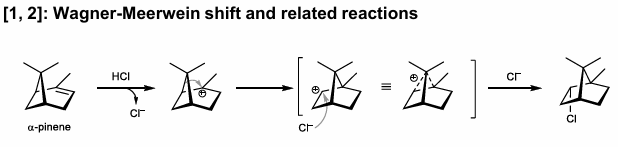
1,2-Wagner Meerwein Shift
1,3-alkyl shifts
Methyl shifts of allylic methyl systems
Stereoinversion required, if a concerted thermal process
Usually occurs with strained systems (vinyl cyclopropane or vinylcyclobutane)
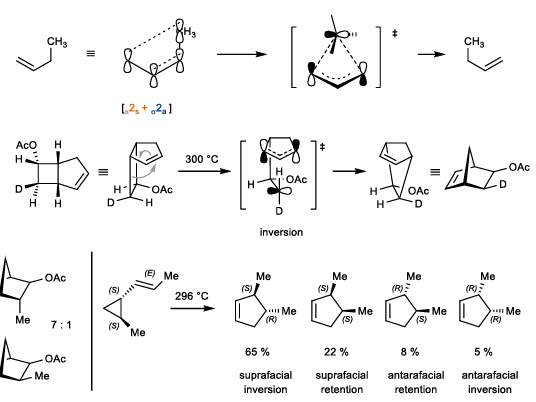
1,5-Hydride and alkyl shifts
Hydride and alkyl shifts of conj. dienes
Suprafacial, stereoretentive
Super fast in strained systems (i.e cyclopentadiene)
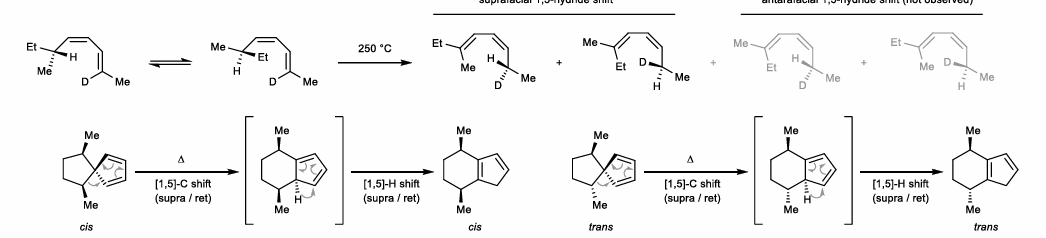
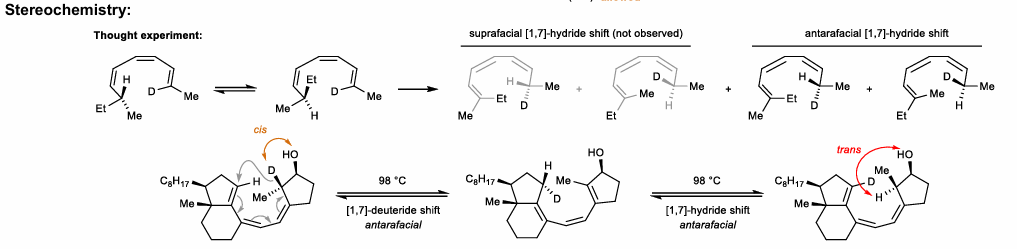
1,7-Hydride shifts
Needs antarafacial component (migration from opposite face)
Allylic shift of conj. trienes (hydride or methyl)
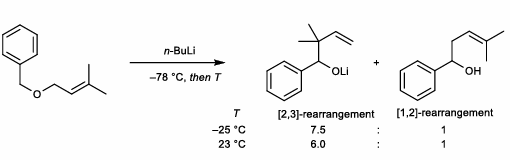
[2,3]-Wittig Rearrangement
Also common with propargylic substrates
Proton alpha to heteroatom but not conj. to allylic group is deprotonated
Still-Wittig
Same concept as regular [2,3] rearrangements, except Li transmetalates with SnBu3
![<ul><li><p>Same concept as regular [2,3] rearrangements, except Li transmetalates with SnBu3 </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/4acd774f-b410-447f-903a-2ab156a5ab89.png)
[2,3]-Wittig Rearrangement Stereochemistry
Always Trans-olefin formed
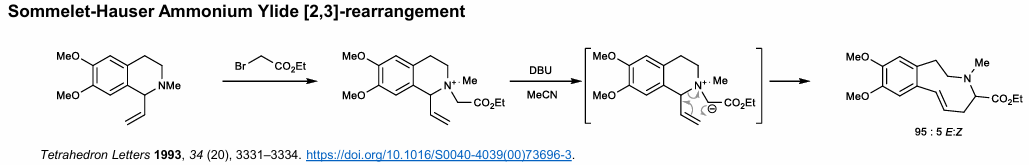
Sommelet-Hauser Ammonium Ylide Rearrangement
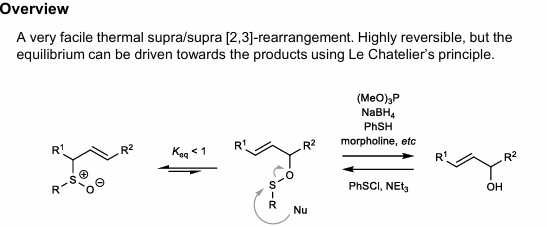
[2,3]-Mislow Evans Rearrangement
Can prepare via Mitsunobu, mcPBA oxidation (1 eq.)
Can cleave with nucleophile (Thiophiles: P(OEt)3, NaBH4, PhSH, etc.) to form alcohol
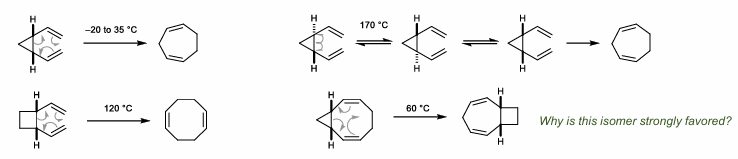
[3,3]-Cope Rearrangement

Strain-Driven Cope Rearrangements
Divinyl cyclopropane, cyclobutane. Remember, ring strain release is about 26 kcal/mol for cyclopropane
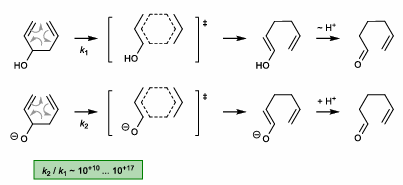
Anionic Oxy-Cope Rearrangement
Anion stabilizes biradical TS
Lower BDE of broken bond
Requires base to form anion (KH, KHMDS, etc.)
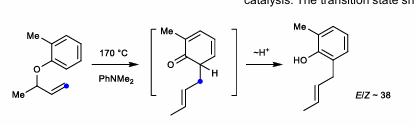
Claisen Rearrangement
Aromatic, alkyl versions. Aromatic is more common
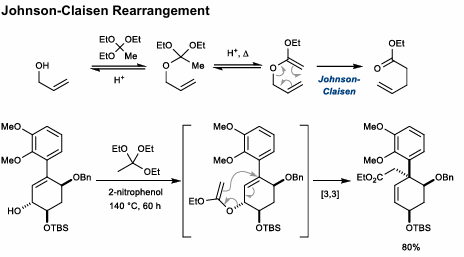
Johnson-Claisen Rearrangement
Use of triethylorthoacetate to form ester after rearrangement
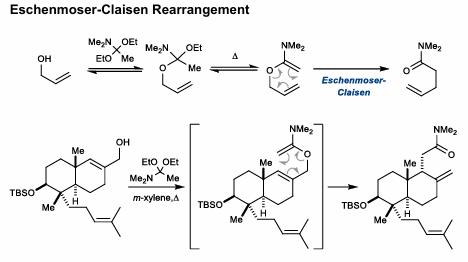
Eschenmoser-Claisen Rearrangement
Forms amide after rearrangement
Ireland-Claisen Rearrangement
Chair TS, stereoselective
Z, E alkenes = trans
E, E alkenes = cis
Deprotonation to enolate, then trapping, then rearrangement

Eschenmoser Claisen reagent
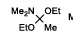
Tandem Aza-Cope Rearrangement

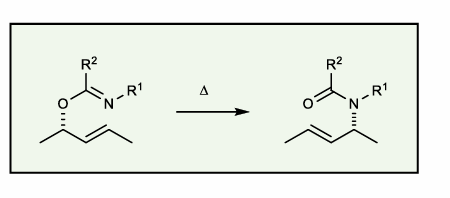
Overman Rearrangement
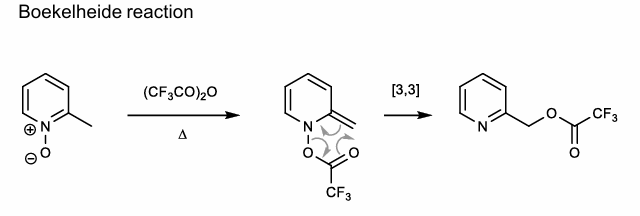
Boekelheide Reaction
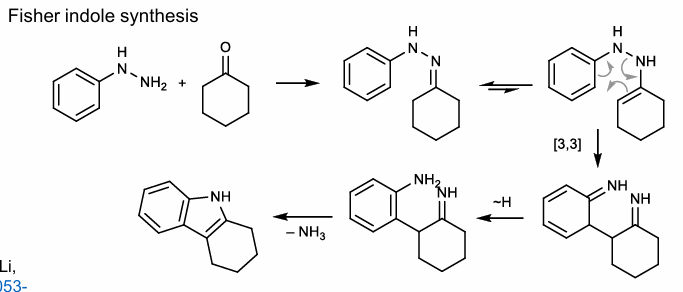
Fischer Indole Synthesis