15 & 16) Redox 1 and 2
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
what do round pyrite grains indicate
no oxygen in water, because pyrite would have oxidized into sulfur
means that no oxygen in water if they got ground up into spheres, and not cubes or a different compound
details on redox processes
major influence on groundwater quality particularly for O2
fate of pollutants in groundwater is often controlled by redox processes
redox are thermodynamically favourable, but most of the time it’s determined by bacteria reacting
oxidation is __
reduction is __
losing = oxidation
gaining = reduction
reaction details: 2Fe2+ + MnO2 + 4H+ <-> 2Fe3+ + Mn2+ + 2H2O
iron is oxidized (2+ to 3+)
Mn is being reduced: in MnO2 Mn is 4+ (oxygen is always -2), then going to 2+, reducing
steps for writing redox reactions
balance elements, except H and O for each half reaction
balance O by balancing H2O
balance H by adding H+
balance charge by adding electrons
subtract half reactions to cancel free electrons and get overall reactions
flip around one reaction and add together to make full reaction
details on theory of redox
expression for change in gibbs free energy relates to produces and reactants of redox
RT/nf is not just moles because electrons have charge
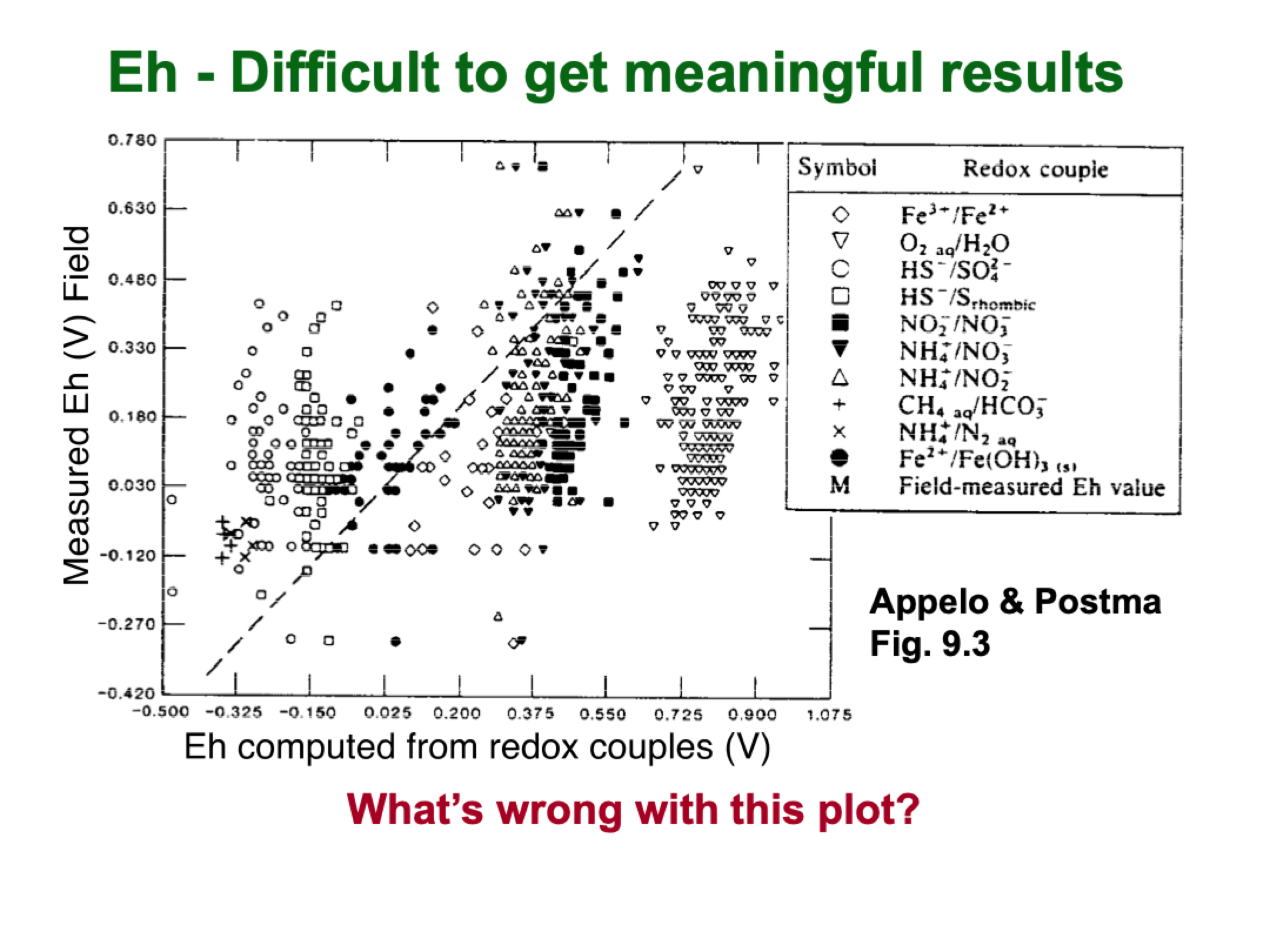
whats wrong with this plot
for white circles and squares, they are consistently above the computed values
if this was actually what’s happening, the calculations would equal reality
the theory does not ever match the reality, so we’re not measuring something meaningful
oxygen is meaningful, we can measure whether water is in eqlm with atmosphere
what is pe
theoretical value, represents the relative tendency of a solution to accept or transfer electrons
analogous to pH (tendency to accept or donate H+)
pe= -log{e-}
{e-} is not expected to exist, can’t really have electrons floating around in solution, but its a way for us to see if reaction will go forward or backward, the drive for a reaction
high pe is what?
oxidizing conditions
pCO2 is always ___ in soils than atmosphere because why
higher
microbes running the reaction
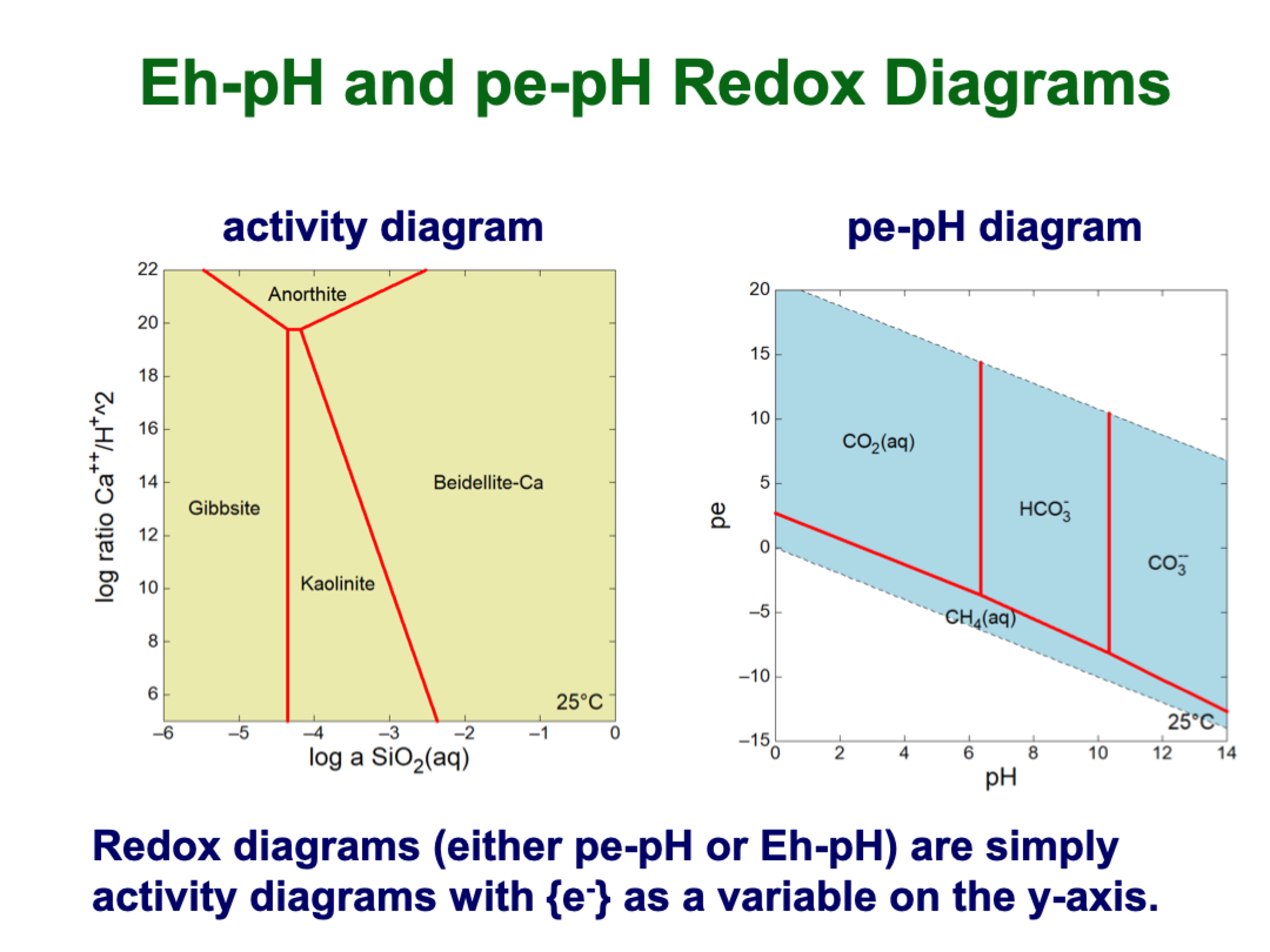
redox diagrams details
at pH zero, pe should be zero for reduction of water to hydrogen
and slightly above 20 at pH 0
slopes are determinable
higher pe is oxidized, so C in upper section is +4
lower pe is reduced, so C in lower section is -4
details on stability of water redox
redox processes require water to allow electron transfer between species;
only reactions stable in water are of interest
H20 <-> 2H+ + 2e- + 1/2O2 is the oxidation limit
½ H2 <-> H+ + e- is the reduction limit
what to do for stability limit equations
lower: choose the value of logK where H2 bubbles out of solution, the easiest value to choose is 1 because log1=0 even though this is not accurate
higher: water cannot exist above the top line, it’s definitely accurate
details on the formation of methane
CH3COOH is acetic acid, typical in freshwater environments
using hydrogen is typical in marine environment
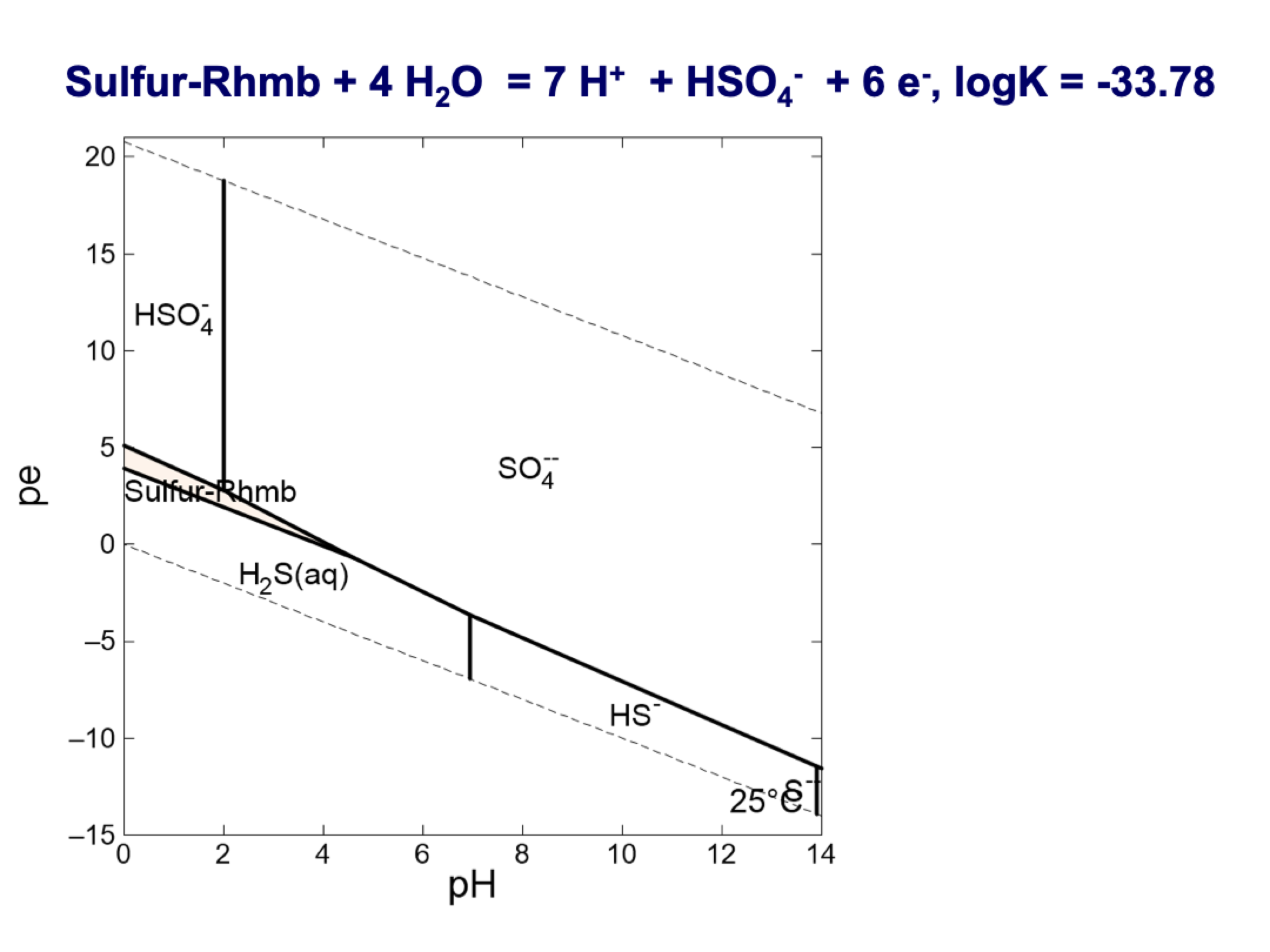
how to do this
write out full K equation with coeffs as exponents
cancel out water and compounds { }=1
take log of both sides writing out exponents as coeffs and adding and subtracting for multiply and division
logK is given
log{e-} = pe
log{H+} = pH
find slope and organize like this:
pe = slope*pH + logK
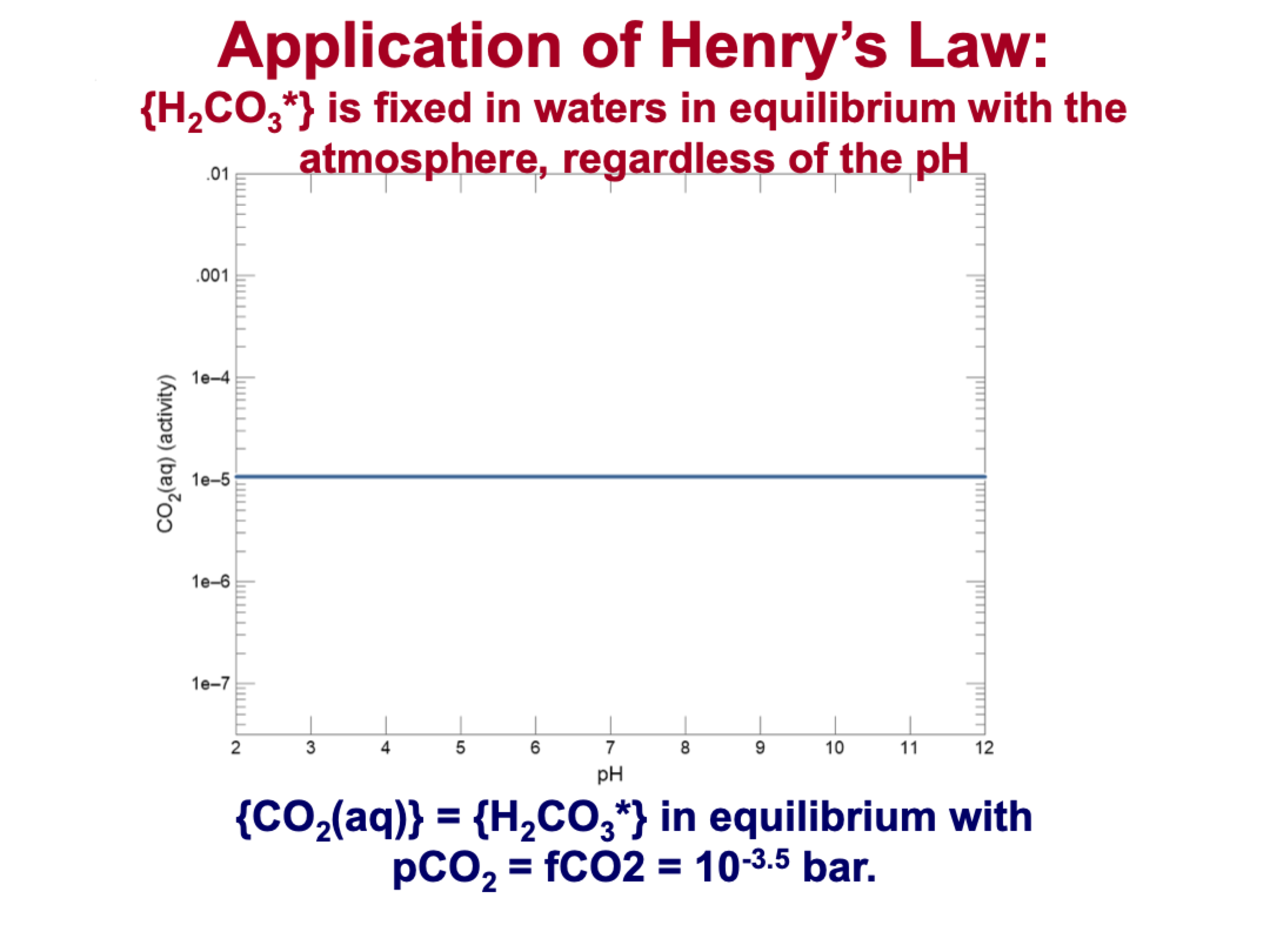
explain this
KH = {CO2 (aq)} / pCO2(g)
KH is constant
pCO2 is exchange with atmosphere (constant)
So {CO2(aq)} is constant
what is ionic strength
measure of total ions in solution, considering molar concentration and charge