MCAT-General Chem/physics
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
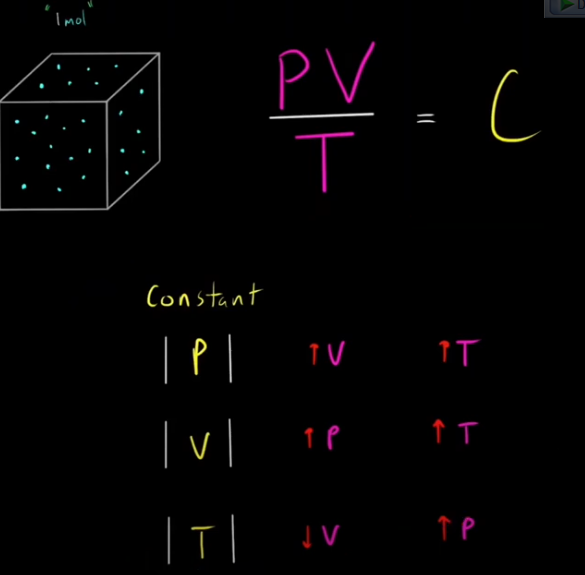
Ideal Gas Law
PV=NRT
When moles in a system is constant... PV/T=Constant (since R is constant)
Memorize photo:
If either P or V is held constant, then numerator/denominator change in same magnitude for C to remain constant. If T remain constant, then variables in numerator (P/V) inversely change compared to each other. For example, T is constant while pressure increase. For C to remain constant, Volume has to decrease in same magnitude.
If we know molar mass of gas molecules in system, we can find density by multiplying molar mass to both sides. PM/RT=density mnemonic pom-rot equals density. (M=molar mass)
Specific heat and calorimetry
Specific heat formula can be used to calculate amount of heat energy used to change temperature of a substance by a certain amount.
Q=mcdt c =J/g*C
In Calorimetry -q=+q, heat lost equals heat gained,
(specific heat capacity is heat (joules) required to raise temperature of 1 gram of substance by 1deg Celsius)
Equals: mc(Ti-Tf)=mc(Tf-Ti)
We can use this to find equilibrium Tf when an object is placed in solution
Latent heat of fusion and vaporization
For non-state change, use typical specific heat eq. (q=mcdt).
For melting of solids, use (Latent heat Formula) q=mL (L is constant) ex 500J=5g x 100J/g
For liquid to gas, use same equation
Also, vaporization cost more energy than melting.
Quantum numbers
Principle- orbital level (n)
Azimuthal-(max range is n-1)-s=0 p=1 d=2 f=3
Magnetic- center orbital is 0
Spin= +1/2 or -1/2 (second will always be negative)
Arrhenius, rate constant, and reaction order
Rate constant, k, tells the rate at which chemical reaction proceeds under specific conditions. For example, larger constant implies faster reaction, and increase in temperature increase the constant exponentially.
K=A*e^(-Ea/RT) Bigger K=faster reaction
Since Ea is negative, therefore increasing denominator would increase K such as T and R.
Lower activation energy means less energy to reach transition state.
Rate=[M]/s
Rate order equation ex.: rate=k[reactants]^(order)
If 0 order, changed concentration doesn't affect rate. If you double first order, rate is doubled. If you double 2nd order, you quadruple, if you double 3rd order, rate is 8 times higher. If you triple 2nd order, you increase rate 9x.
Order is found experientially by comparing initial rate of reactions under different concentrations. Coefficient has no correlation to reaction order.
Le Chatelier’s principle
System responds to restore equilibrium concentrations. If exothermic, heat is released. If endothermic, heat is required. In an endothermic example in which heat is decreased, reaction is pushed towards reactants (left). In exothermic example in which heat is decreased, reaction is pushed to right (products)
H2O autoionization
H2O+H2O=(H30+)+(OH-)
Since we know pH of water is 7, then [H3O+] and [OH-] are both 1 x 10^(-7) while water product constant Kw is 1 x 10^-14
Kw=[H3O+][OH-]=1x10^(-14) When neutral
Btw remember... pH=-log[H30+] and pOH=[-OH]
So... pKw=[pH]+[pOH}
Strong acid Ka disassociation
With strong acids all moles of strong acids are converted into hydronium
Keq=Ka but for acid example ka=[10,000]/[1] in which hydronium is product
6 strong acids: HCl, HBR, HI (HI stronger because I is larger and dissociation energy is lower than Cl), HNO3, H2SO4, HClO4 (perchloric acid) (Mnemonic for last 3, PNS)
Ksp solubility and ion product
Similar to Keq equations but ignore solids and its called KspQ is used when not at equilibrium.if Q
If Q=Ksp, then Keq duh
Q=IP, Ion product (IP) is the same as Q (not at equilibrium) as Ksp is to Keq
Molar solubility. To find unknow concentrations ion products, example:Ca(OH2)=Ca2+ + 2OH-Ksp=6.2 x 10^(-6)
Then 6.2 x 10^(-6)=[x][2x]^(2)
6.2 x 10^(-6)=[4x^2]
x=0.116M
Gibbs free energy
If standard state delta G is negative (releasing), then Keq>1. If it’s positive, then Keq
electrochemistry
Galvanic cell:
Cathode is where cations (positive) gets reduced and is positively signed. Anode is where anions (negative) gets oxidized and signed negative. (an Ox. Red Cat). Greater reduction potential gets reduced while greater oxidation potential gets oxidized.
Ecell=redpot+oxpot.
+Ecell = -G, spontaneous
-Ecell = +G, nonspontaneous
Electrolytic cell: Battery is added in circuit to reduce electrode with lower reduction potential instead. Cathode is negative here, while anode is positive.
Salt bridge allows e- to continue to move to cathode. Without, salt bridge, e- will eventually move in reverse as the cathode would become more negative charged than the anode.
As this reaction progresses, more solids will form on cathode while anode will lose solids. This works until salt bridge is depleted out the initial salt ions.
Advance determination of reactants/products
Determine the acids and its conjugate for stability of each side. Then, you can use equation pkaL-pkaR=pka. Use this pka to find Ka and find product reactants ratio.lower the pka, the stronger the acid. Lower pka, means higher ka - greater ratio of product over reactants
Trigonometry: Unit Circle
Sin90=1, Sin0=0Cos90=0, Cos0, 10=(1,0)30=(sqrt3/2, ½)45=(sqrt2/2, sqrt2/2)60=(1/2, sqrt3/2)
90=(0,1)
Kinematics
Displacement formula: Xf-Xi=Vo*t+1/2at^(2), simplified equation if initial velocity is 0 would be d=1/2a(t^2)
Final velocity equation: Vf^(2)=Vi^(2)+2ad or preferred Vf=sqrt(Vi^(2)+2ad)Think dropping ball example
Everything Work
W=∑FdFor angles, you can find component which I prefer. However, otherwise use the equation W=∑Fd*costheta (this is angle between force and direction of motion)
This is to find how much work that a force did on an object. Check notes.
Uniform circular motion(centripedal)
Centripetal acceleration: ac=v2/r
Centripetal Force: Fc=m*v2/r (basically F=ma)
For circular motion, involves centripetal force perpendicular to tangent velocity of moving object.
Pressure in fluid system
Gauge pressure: Pressure (P)=p(density of liquid) * g (gravity) * h (distance from surface to point of interest, lower means more pressure as there is more mass applying pressure above the point)
Pt=Pg+Patm ---- To determine absolute pressure we need gauge pressure and atmospheric pressure. Atmosphere pressure make sense at air applies pressure and it has a density. Unless the situation is of a closed container, then you have to consider atmospheric pressure that is also applying pressure to the liquid. Standard units (SI unit) for pressure is Pa-Pascals.
Ohm’s law
V=IR
Units: R=Ohms, I=ampIn series, we add resistance of resistors together
In parallel, use (R1xR2)/(R1+R2) or 1/R1+1/R2=1/Req
Function of resistors is to limit current
R=pL/A
Length on numerator increases resistance, while cross-sectional area on denominator decreases resistance.
Resistors also make drops in voltages. Positive lead to resistors makes the first drop (difference in total minus that resistance). By the time you pass last resistor, the area between that point and the negative lead should be 0V.
To calculate the voltage drops, you need overall current first. You can find overall current (AMP) after adding all resistors. To find voltage drop, you can apply ohm’s law for each resistor in series. If parallel section, you apply ohm’s law over the total resistance in parallel. You solve for voltage with the overall current and the resistance at each point (used combined resistance for parallel sections); this is how voltage drops for each point of resistor.
In series, the current is the same. Also, for resistors in parallel, the current diverges between each resistor and come back together to make up total overall current.
Also, voltage is difference in electric potential.
Sound: Decibel and Intensity
Sound is vibration of air molecules. Velocity of sound can be found through these 2 eq.V=sqrt(B/d) or v = f λ V=freq x wavelength----freq (1/s or Hz), wavelength (m).B=bulk modulus of medium, d=density of medium
Intensity- I=W/m^2 ---- power (J/S or watt) moving through surface area
Loudness – measured in decibels – easier than using magnitudes from intensity.Basically intensity measures how much energy pass through a cross section per second. Intensity decrease the further it is from the source because the area (sphere) in which the sound spreads through is larger. DB=10log(I/I0) and I0=1x10^(-12 )W/m2------ (constant in which lowest intensity a human can hear)Decibel (loudness)- To explain more easily how many magnitude DB greater one intensity if from another… Honestly, it’s just a better number representation of number for intensity. Again, used to describe order of magnitude difference of intensity between two sounds.
Example 1: One Intensity (I)=50 w/m2, while the other source is I=5000000 w/m2. In this case, the order of magnitude difference is 5. Multiply by ten, and you get a decibel of 50dB. Therefore, the latter sound is 50dB louder. This explains how the equation above is used. Count zeroes and figure what the magnitude difference is between the two. Order of magnitude times 10 equals decibels difference.
Example 2: If the two sounds have intensity of 3 w/m2 and 600 w/m2, we will find order of magnitude between 30 and 3000 as this is where 600 lies between. This gives you 2 and 3 order of magnitude greater than 3 w/m2, so the answer is 20-30dB difference. Trend: further out increases surface area. (Think sphere) ... so lower intensity. In addition, more air resistance (attenuation) causes lower intensity as well.
Doppler Effect
f'=f[(v+/-vd)/(v+/-vs)]f' is frequency perceived by detector
Ex. If the source is moving away from the detector at 50m/s and the source is moving towards the detector at 150m/s, then the denominator will use positive sign as this decreases frequency and the dominator will use positive sign as this will increase the frequency.
Coulomb’s law, electric field, electric potential and electric potential energy
Coulomb’s law, magnitude of force between two charges. K=9x10^9 Nm^2/C^2F=kq1q2/r^2. Kind of similar to Newton’s 3rd law, Force of gravity equation Electric field is created by every charge and eq:Ef(N/C)=kq/r^2Force felt when charge is placed in EF. à F=kq1/r^2 x q2
Electric potential
J/C=kq1/r
Remember that Voltage, V=J/C, region of electric difference/electric potential – characteristic of region of space. EPE is a system of energy if you add in a charged particle in EP region.
Electric potential energy
J=kq1q2/r x q2
photoelectric-effect:
The work function is the energy needed to remove an electron from a metal. When there isn’t enough energy, a photoelectron won’t be produced.
eq: Ephoton=Wwork function+KE
So Ephoton>Wwork function to release photoelectron, input energy has to be greater than output energy
Law of reflection
Snell’s law:
Angle of incidence light=angle of reflection for refraction use this eq
n(i)Sintheta(i)=n(r)Sintheta(r)
the side with the greatest angle of refraction will have smaller angle compared to normIf both sides are same medium, both angles are the same.
When light enters a material with higher refractive index, the angle of refraction will be smaller than the angle of incidence and the light will be refracted towards the normal of the surface.
Mirror and lenses
1/f=1/do+1/di
do=distance from mirror/lens to object. di=distance from image to mirror. f=focal length is calculated as one-half radius of curvature from mirror or lens. Focal point is the point on axis of lens in which parallel rays converge to or from which diverge from. Because a diverging lens spread light rays instead of focusing them (converging), there isn’t really a focal point. However, by tracing the lights backwards from the diverging rays, you can find a virtual focal point on the previous side. Hence, the focal length of a diverging lens is negative, creating a virtual image. Also, focal point exists on either side of the lens, but whether it’s negative or positive depends on the nature of the lens (concave/diverging or convex/converging)+f for converging and convex lens/mirrors
-f for diverging/concave lens/mirrors
do always positive – as it’s just distance from object to lens
+di for images that makes sense (behind lens) (only convex lenses)
-di for images that doesn’t make sense (same side as object) (either convex or concave lenses; concave always have negative di)
magnification equation:-di/do=M or hi/ho=M, (h is height) (M=magnification)M>1, magnifiedM
Nuclear radiation vs electromagnetic radiation
As elements gets bigger, there ratio of neutron to proton must be greater than 1:1 for stability (more neutron compared to protons).Nuclear radiations (what’s being emitted/released from these conversions):β−-emission- balances neutron becoming proton (neutron losing some negative particles such as electron to become proton)
β+-emission- balances proton becoming neutron (proton losing some positive particles such as positron to become neutron)
α-emission- removes proton and neutron
Gamma rays are both magnetic and EM that’s nuclear radiation Barium-137 can cause cancer
Magnetic field
Equation for what magnitude of force a charged particle feels in a magnetic field:
F=qVB q=charge (coulomb), V=velocity m/s, B=strength of magnetic field (T-tesla)Since V and B are vectors, full equation is F=qVBsintheta
Direction of magnetic field is always from north to south.
To find direction of Force. Use right hand rule for positive charge and left hand for negative charge.
Thumb for direction of charge, fingers for direction magnetic field. Where palm face is direction of force.
Torque
T=Fd or more accurately T=Frsintheta. d=sintheta. At static equilibrium, net torque is 0. And there is no acceleration in any direction
SI units- power of tens prefixes
Terrible gigantic monsters killed one million men napping peacefully
terra, giga, mega, kilo, centi, milli, micro, nano, pico
^12, ^9, ^6, ^3, ^2, ^-3, ^-6, ^-9, ^-12
Dalton's Partial Pressure& Henry's solubility laws
Dalton’s Partial Pressure law:
Mol fraction (molx/molt)*total pressure=partial pressure (x is gas of interest while t is total moles of all gasses. Ex pressure unit=atm)
Henry’s solubility law: molarity=solubility constant x Partial pressure
Ex: M=(M/atm) x atm
(Molar per atmosphere) (solubility constant of gas in particular solution)
Trends: changing solvent will change the solubility constant. Doubling Partial pressure will double molarity.
Keq and Q quotient
Keq={[products]^(quotient)}/{[reactants]^(quotient)}
Q is same shit but not at equilibrium If Q is less than Keq, want to move towards Keq (form more products). If Q is more than Keq, move towards Keq (move towards reactants).
If Keq=1, then equal amounts of products and reactants at equilibrium. No free energy at Keq, free energy is released when Q
Titration- acid/base
Henderson Hasselbach: pH=pka+log[conjugate base/weak acid) - use pka of that weak acidif weak base then pOH=pkb+log[conjugate acid]/[weak base] - use pkb of that weak acid.
pH=pka at ½ equivalence. The weak acid acted as buffer, prevented drastic pH when OH- (strong base) was added. After equivalence, pH increases drastically.
Weak acid dissociation
Weak so ka would be for ex =1/10,000 and would need ICE table to find H3O+ concentration at equilibrium.
So usually this calculation comes to Keq=[x^(2)]/[reactants concentration]example, if we start with 1M reactants of weak acid then eq is Ka(1/10,000)=[x^2]/[1-x]. but since x is so small anyways as its weak acid, we can ignore it and get(1/10,000)=[x^2]/[1]. There, x^2 is from multiplying [conjugate base] and [hydronium]By finding [H3O+], we can find pH
Ka/pka and kb/pkb
-logpka=ka and logpkb=kblarger kb=stronger base, smaller pkb=stronger acid etc.
Multiplying ka x kb of conjugate acid/base pair will equal=1x10^(-14)
Adding pka+pkb=14
Btw remember... pH=-log[H30+] and pOH=-log[-OH]
Work, Force and Power
F=ma in which m=kg a=m/s^2 and F is N for newtons
W=F*d in which distance is in meter and W is J for joules
Power=W/s or J/s and Power is W for wattage
Position, velocity, acceleration vs time
Area under velocity vs time, will give distance travelled. Area under acceleration vs time, will give change in velocity. This is kind of like taking integral (one step back)
Newton’s 3 laws of motion
1) Law of inertia (inertia-tendency to maintain current velocity. More mass=more inertia and required more force to change velocity)
2) F(net)=ma. Ex. Accelerating a heavier object requires more force. Same force applied to different weight objects will see the heavier object accelerate less.
3) Equal but opposite force F(gravity)=Gm1m2/r^(2) ->r is distance between the two masses
Also, equal and opposite forces are never from same object. For example table to earth and earth to table forces. G=6.6710^-11 N(m^2/kg^2) or 6.6710^-11 (m^3/(kgs^2) Advance Newton's 2nd law F=ma calculations with vectors: see notes
Law of Conservation of energy with GPE (gravitational potential energy) and KE (kinetic energy)
PGE (potential gravitational energy)=mgh (potential work in joules). ---Energy difference relative to a point. Similar to work as its W=ma*dAs work is done, it’s converted into kinetic energy as energy is never destroyed. Eq for KE=1/2mv^2With this equation and type of problem we could find velocity.
How we should solve: total potential energy (initial) = GPE+KE
Frictional Force
Friction=opposes motion
>static=prevents stationary object form moving
>Kinetic=slows down moving object
Coefficient of friction= tells much something has to work to resist something moving on that object, represented by letter u
us-tells how much something will resist an object starting to move.
uk-how strongly an object will resist something already moving
us>uk ; much more difficult for an object to start moving than continue moving (having momentum).
Force of kinetic friction:Fk=uk*n
Force of static friction:Fs=us*n
n=normal force
(Fn)Free body diagram:
NetFy=0=Fn-Fg
NetFx=0=Fapp-Ff (Ff=Force of friction, Fk or Fs) (Fapp=applied force)
Simple harmonic motion
About finding angular frequency (oscillations/radians per unit of time) and understanding its components.
Swinging pendulum; Mnemonic: wiggleω=sqrt(g/L), g=gravity, L=length of pendulum
Oscillating spring; Mnemonic: wack 'emω=sqrt(k/m) k=hook's constant, m=mass
Unit not important, but knowing how the factors affect the angular frequency.
Force of buoyancy
Volume of object submerged equals displaced water. If object is denser than fluid, it will be full submerged. If less dense than fluid, it’s partly submerged. For ex. If 0.0006m^3 volume of ball is submerged in water, then volume of water displaced is also 0.0006m^3. Ball is stationary at equilibrium. Ball is feeling two forces, force from weight of ball and weight from water displaced. Therefore Force(weight of ball)=Force(weight water displaced).use bowling ball as reference (object)
First, think F=ma or F=mg, so… We can do mg=mg or (ball)pVg=pVg (water)---- in which m=pv, remember density(p)=mass/volume.
pbVbg=pwVwgAs gravity is constant on both side, we can simplify to (ball)pV=pV(water) pbVb=pwVw
Final derivation:pb/pw=Vw/Vb, remember Vw is volume of water displaced by ball
This tells us ratio of density of ball to water = percentage/ratio of volume of ball submerged. Multiply right side by 100 for %. Density of object divided by density of fluid it falls in = percentage of object submersion.Again, the above only counts for when object is less dense than the fluid.
Buoyancy is the force of liquid wanting to lift object upwards. Which is equal to mass that the water got displaced by.
Basically, density of object divided by density of fluid equal percentage submersion of object in the fluid
Bernoulli’s principle
Bernoulli’s principle: P(pressure)+1/2pv^2+pgh=Energy(total)or
Pressure + kinetic energy + gravitational potential energy = Energy(total)
Energy at any given point is the same. For example (point 1)P+1/2pv^2+pgh= P+1/2pv^2+pgh(point 2)
Energy 1=Energy2
continuity principle: (point1)AV=AV(point2) if Surface area is 10 times smaller at point 1m then velocity at point 2 is 10 times smaller. Reason is flow at point 1 have to be same at point 2 as flow is same everywhere. This is similar to conservation of mass law.
Smaller area, then greater velocity, then greater kinetic energy. Check notes if need help
poiseuille's law
Flowrate is directly proportional to pressure difference at each end of the pipe. It is inversely proportional to viscosity of the fluid and length of the pipe. f(m3/s)=( πΔPr4)/(8uL)
Capacitance
Q=VC
Capacitors store charge from voltage source.C=EA/d--- greater distance between capacitors, lowers capacitance. Greater SA of capacitors increases capacitance.This is kind of reverse the trends happening with resistors.
(The more positive an area is, the higher the electric potential is for electron to move this way)
How much charge stored on Capacitor: VC(farads)=Q (Coulombs). The greater the capacitance, the more charge (coulombs) is stored in the capacitor. If a set of capacitor plates store 100C, then its +100C on one plate and -100C on the other plate.
Voltage times farads equals charge (coulomb)
In series, use (C1xC2)/(C1+C2). For parallel, add together ----- opposite of resistance.
Also in series, capacitors store same charge. In parallel, the charge diverges and adds up to same overall charge.
When it comes to finding volage drops, it’s similar to above method for resistors, except you use VC=Q equation instead and solve for V. First you find overall capacitance. Using overall voltage, you can find overall charge (similar to how we found overall current for resistor problems). Knowing overall charge, we can solve for voltage through each individual resistor. For parallel capacitors. You solve for voltage using the overall capacitance (similar to how you use overall resistance for ohm’s method).Really, the only difference in logic is the trend of object in parallel and in series.
Electromagnetic radiation
Gamma, x-ray, uv, visible, infrared, radio visible-400-700nm
E(energy/photon) = h x f (h is Planck's constant= 6.62610-34Js)
V=frequency x wavelength
All ER have velocity of 3x10^8m/s
1st law of thermodynamics
Internal energy=UU=w+q ---work and heat
Contributions to internal energy: kinetic energy, rotational energy, vibrational energy and electric potential energy.W=Pressure x delta volume
Compression is work done while expansion is work lost.PV graphs trends, think of the previous lines. Area underneath represent amount of work done or lost.
Common pKa values
Ammonium, NH4+: 9.25 weak acid
Ammonia, NH3: 35 weak base
HCL: -7
Hydrogen Cyanide: 9.2
Weak Acid/weak bases identification
Weak Acids:
Any acid with formula H and an anion.
For ex. HF and HC2H3O3 (Acetic acid).
These starts with H. Or they end with COOH. Ex. acetic is also written CH3COOH.
Weak Bases:
And base with amino group, NH2, or variation like N or NH.
Example: N2H4 or CH3NH2.