CHEM 1211 Section 2.2 (Nuclides and Their Symbols)
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
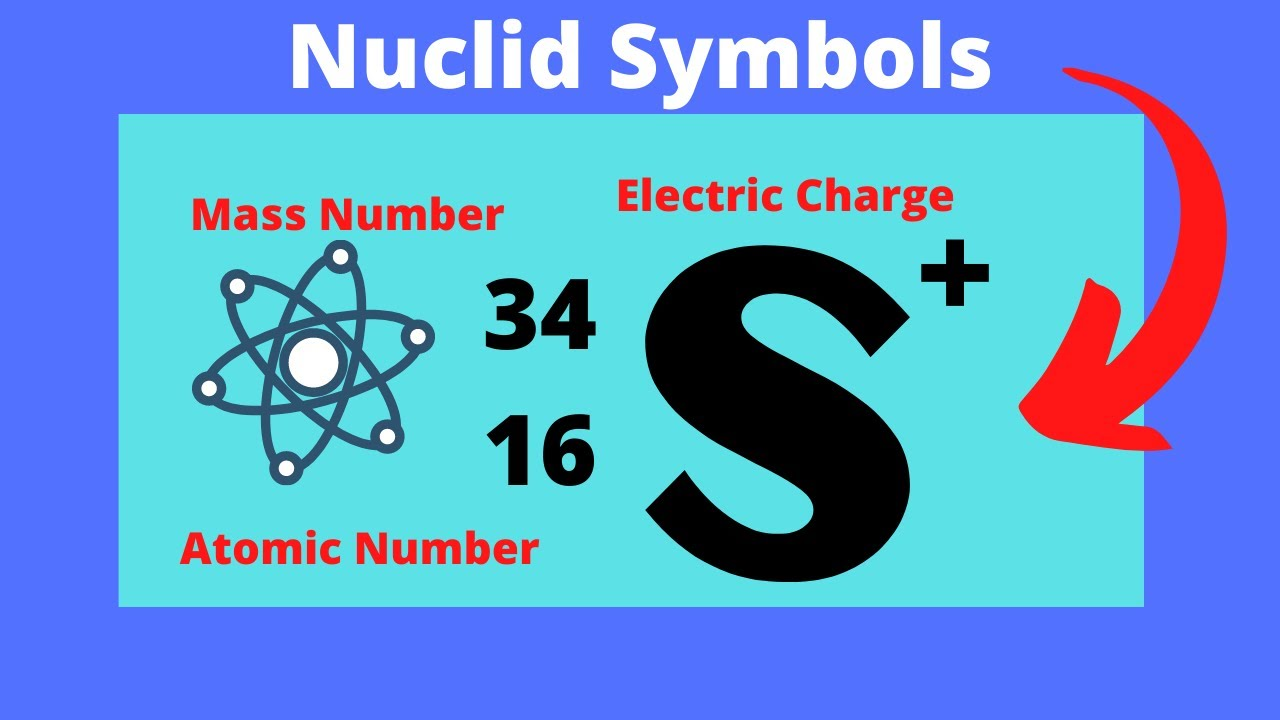
Nuclide
A species of atom characterized by the number of protons (atomic number, Z) and neutrons (mass number, A)
Isotopes
Atoms of the same element that have the same atomic number but different mass numbers due to varying numbers of neutrons.
Atomic Number (Z)
The number of protons in the nucleus of an atom, which determines the identity of the element.
Mass Number (A)
The total number of protons and neutrons in an atom's nucleus.
Ions
Charged particles formed when atoms gain or lose electrons.
Anions
Negatively charged ions that have more electrons than protons.
Cations
Positively charged ions that have more protons than electrons.
Subatomic Particles
Particles that are smaller than atoms, including protons, neutrons, and electrons.
Nuclide Symbol
A representation of an atom that includes the element's symbol, atomic number, mass number, and charge.
Periodic Table
A tabular arrangement of elements organized by increasing atomic number and similar properties.