8. Chirality 1&2
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
What parts of drug processing does chirality impact?
- Absorption
- Distribution
- Metabolism
Define stereoisomers (also called optical and chirality)
- Isomers with the same molecular formula and same connectivity of atoms but different arrangement of atoms in space.
Define constitutional isomers (also called structural isomers)
- Isomers whose atoms have a different connectivity.
Define enatiomers
- Stereoisomers that are non-superposable mirror images of each other.
Define diastereomers
- Stereoisomers that are not mirror images of each other
What are 6 key points about constitutional isomerism? (Structural isomerism)
1) They are molecules with the same formulae but different connectivity of C skeleton.
2) The isomers have different physical properties (e.g. m.p, b.p, solubility, density).
3) The isomers have different chemical and pharmaceutical properties.
4) The atoms and groups are often arranged differently.
5) Different connectivity of atoms can resulting diverse functional groups in isomers, even though they have the same formulae.
6) Constitutional isomers cannot interconvert.
What are 3 constitutional isomers of C9H8O4?
- Aspirin
- Caffeic acid
- 4-Hydroxyphenyl-pyruvic acid
What is geometric isomerism?
- Same formulae, same connectivity of C skeleton, but have different spatial arrangement of substituents around alkene.
- There are two substituents on opposite ends of pi bond, i.e. cis and trans.
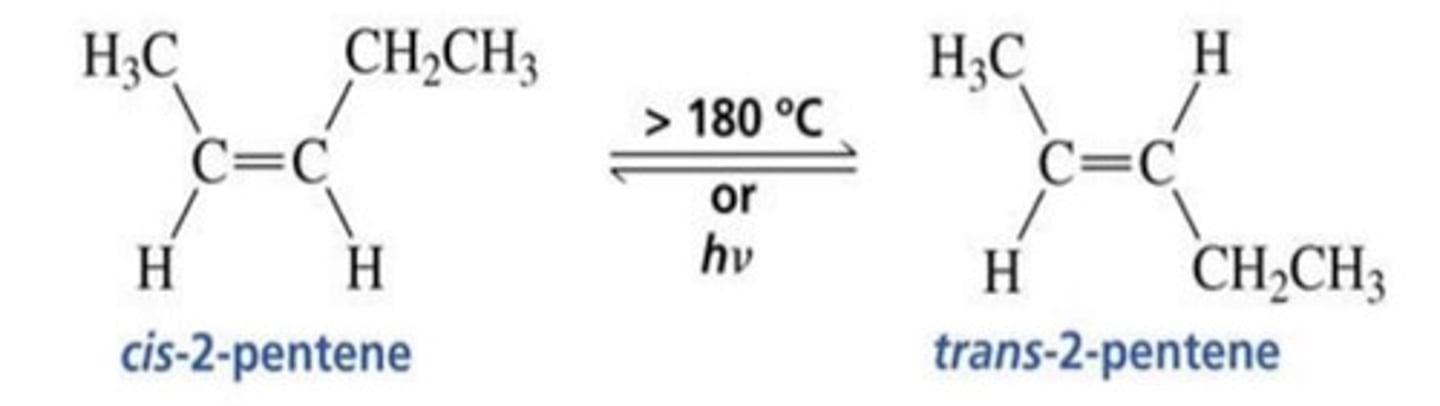
How do we decide if something is cis or trans?
- Using Cahn-Ingold-Prelog rules.
How do we know what substituent to prioritise to work out whether the alkene is cis or trans?
- You decide based on the atomic number of the atom directly attached to the sp2 C.
- The higher atomic number is prioritised.
When is an alkene cis?
- When both of the priority groups are on the same face of the alkene (also known as Z).
When is an alkene trans?
- When both of the priority groups are on opposite faces of the alkene (also known as E).
Why is geometry important using the e.g. of Tamoxifen?
- The Z (trans) geometry of tamoxifen gives it the correct shape to bind tightly to the estrogen receptor causing inhibition of the signalling that would result in cell growth.
- In this way, breast cancer cell growth can be inhibited.
Why is E-Tamoxifen not beneficial compared to Z-Tamoxifen?
- E-Tamoxifen has the incorrect size and shape, so has no binding and no inhibition of estrogen binding.
WHEREAS,
- Z-Tamoxifen is complimentary in shape and size and can efficiently block the estrogen receptor.
Why are stereoisomers not interconvertible?
- As they have a fixed geometry.
What do stereoisomers usually arise from?
- Usually arise due to asymmetry around a saturated carbon atom (4 different groups).
What makes something chiral?
- Usually occurs as a result of a chiral carbon.
- These molecules have no internal plane of symmetry.
- The mirror image of the molecule is non-superimposable.
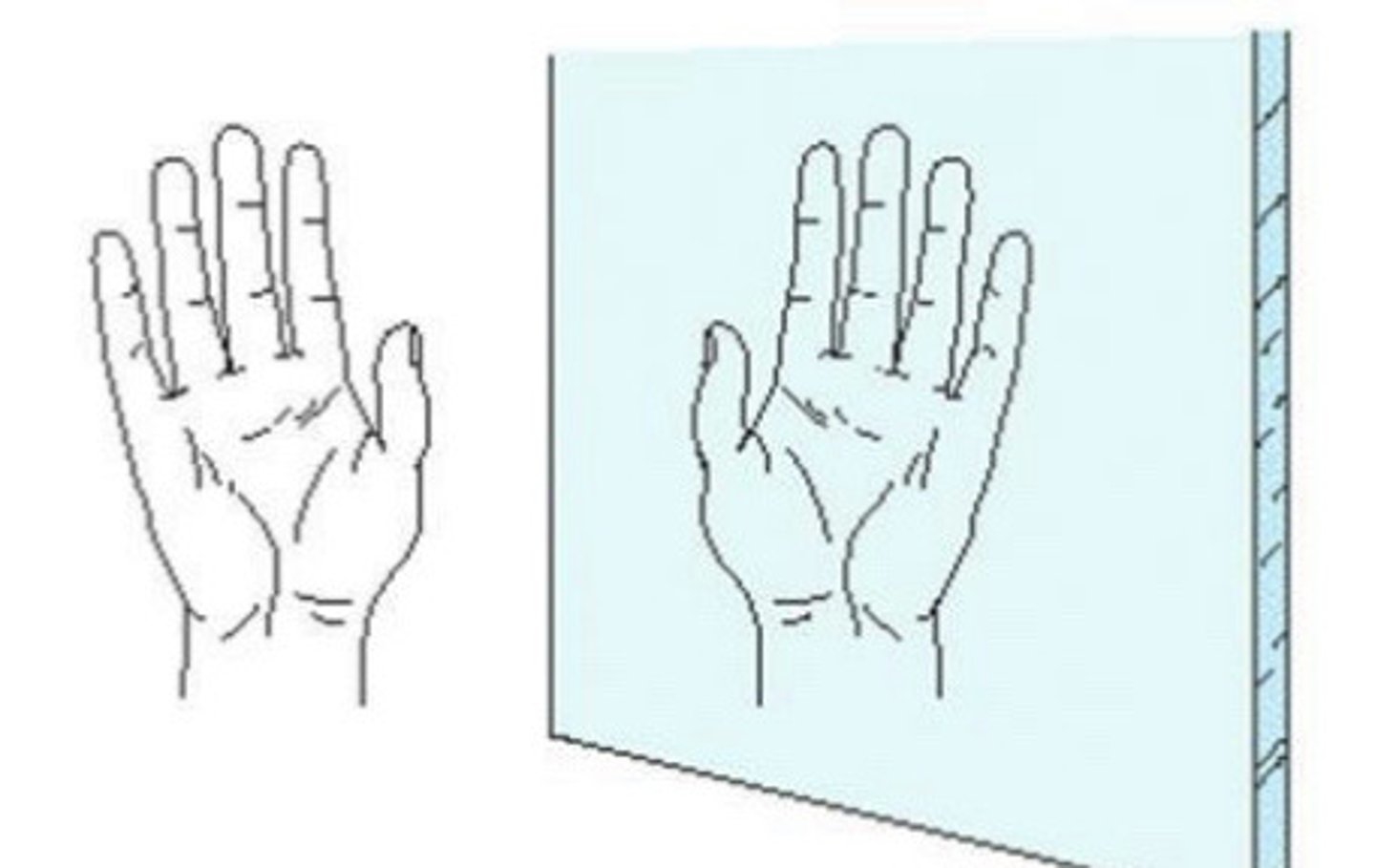
What is normally the hybridization state of a chiral carbon?
sp3 hybridized.
How many groups or atoms does a chiral carbon have attached to it?
- A chiral carbon has 4 different atoms or groups attached to it.
If a compound is superimposable, what is it?
- It is achiral (not chiral).
If a compound is non-superimposable, what is it?
- It is chiral.
What are the non-superimposable mirror image forms called?
Enantiomers
In a chiral pharmaceutical, how do we refer to the different forms?
- We can refer the mirror image forms as either R or S.
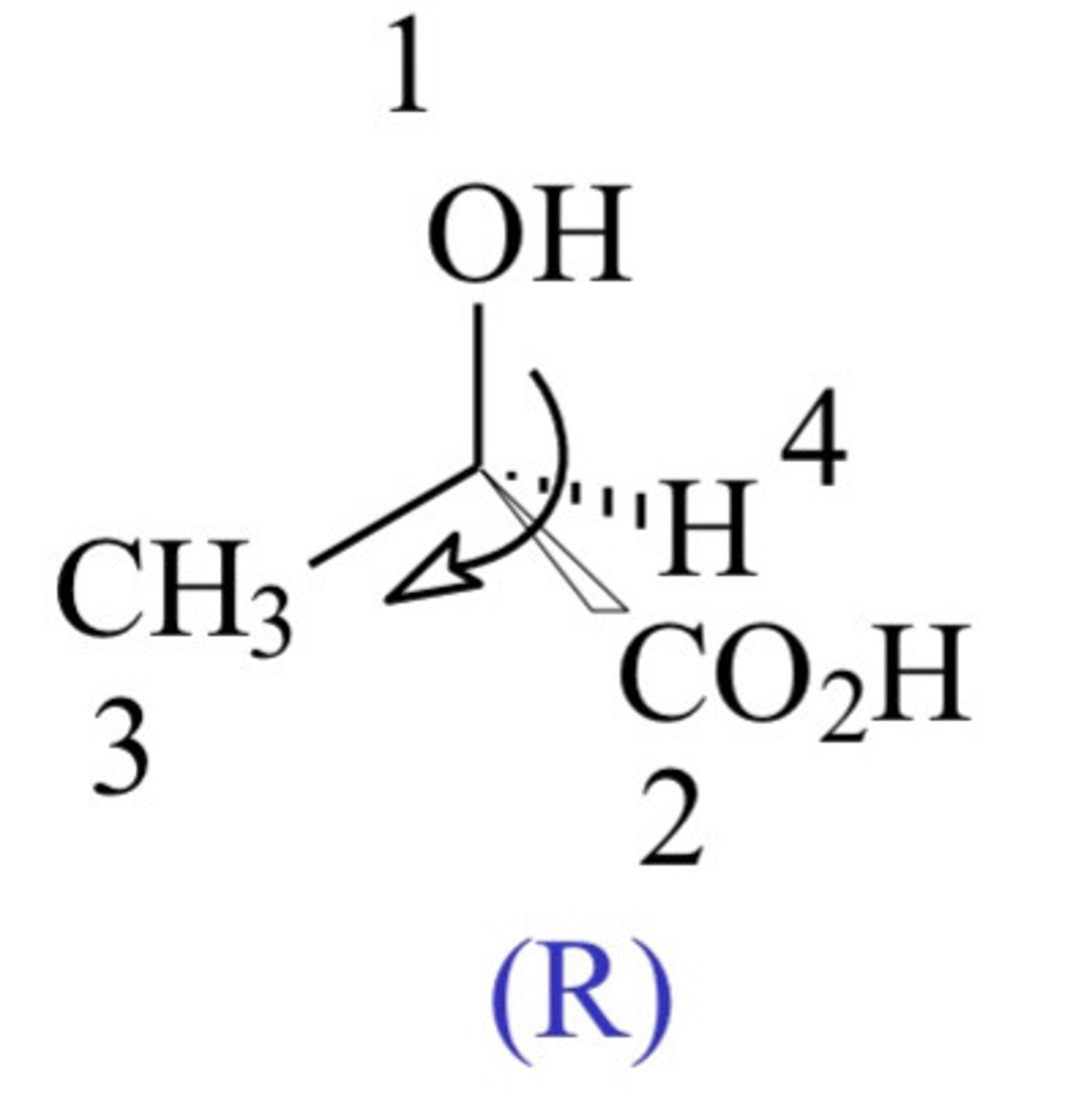
When would we refer a chiral compound as R?
- When the priority groups are going in a clock wise order, i.e. 1 - 4.
(If order 1 -> 2 ->3 clockwise, classify as R)
When would a we refer a chiral compound as S?
- When the priority groups are going in an anticlockwise order, i.e. 1-4.
(If order 1 -> 2 -> 3 anticlockwise, classify as S)
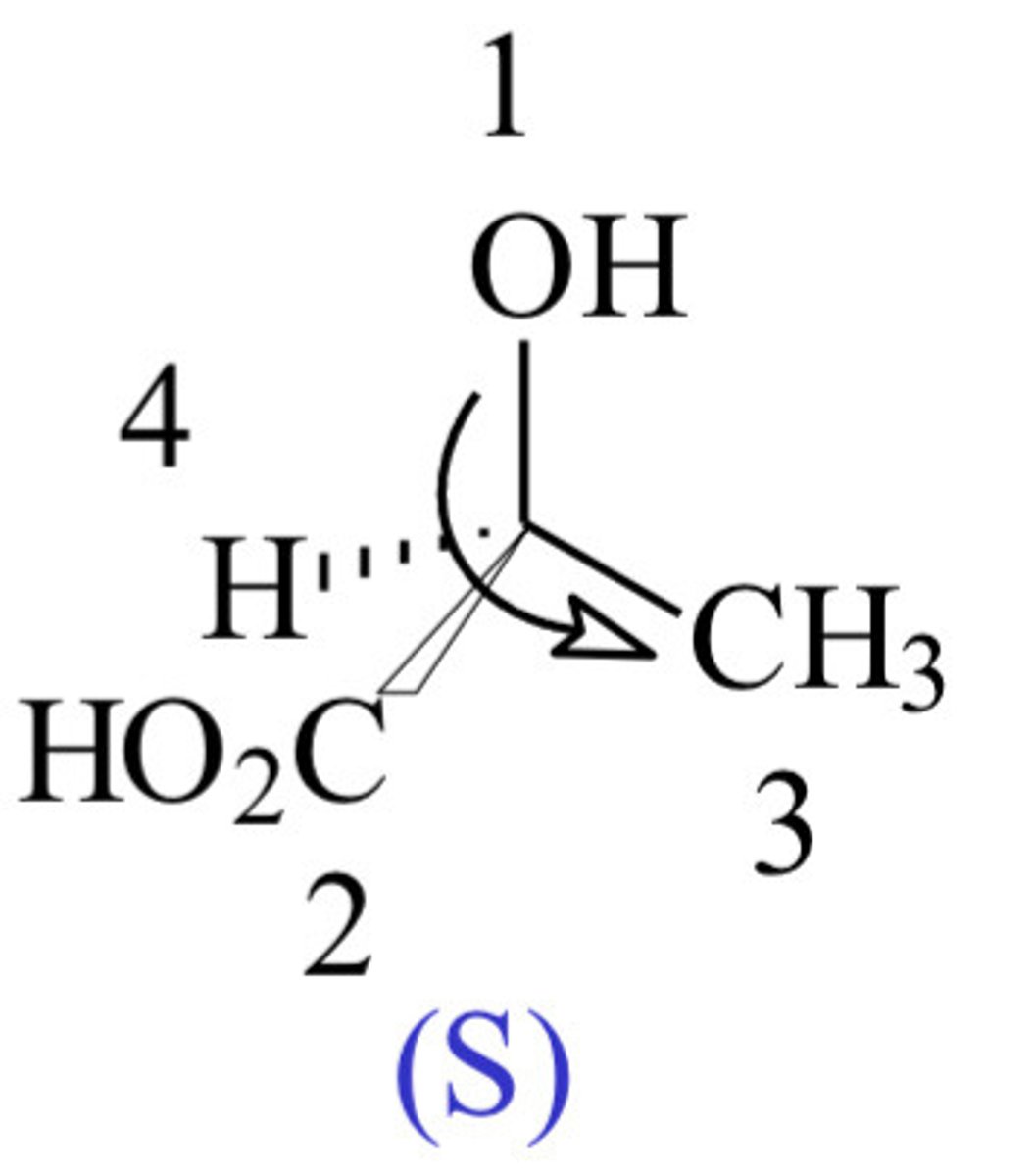
Which atom in a carbon compound should be on a dashed wedge bond?
- The one with the lowest priority, i.e. priority 4. (Should be at the back on a dashed wedge bond).
How do you know which atoms to prioritise when assigning either R or S?
- Prioritise atoms directly attached to the chiral carbon using atomic number
- If atoms attached to the chiral carbon are the same, look at the next atoms attached until you find a difference.
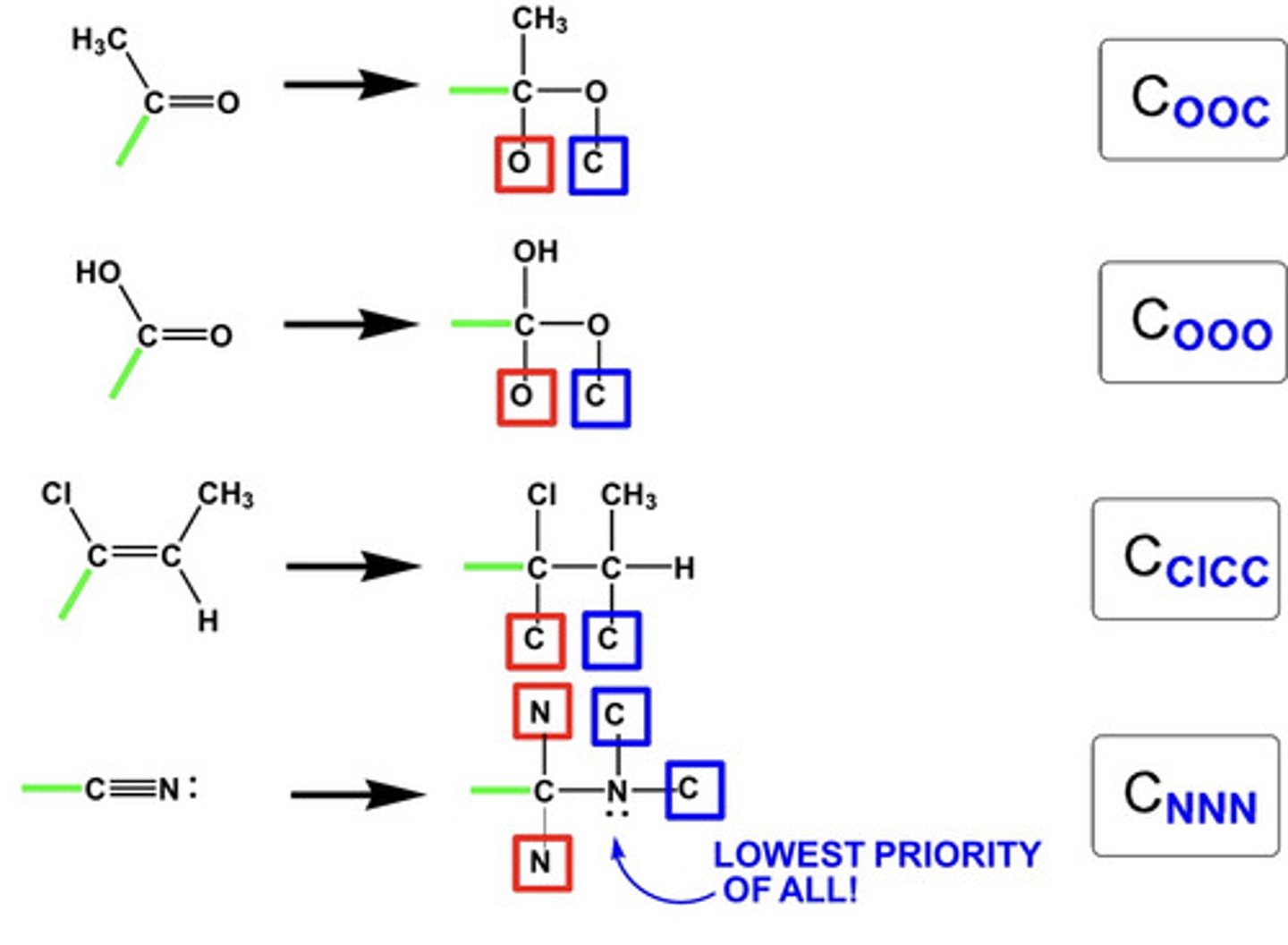
What does the Cahn-Ingold Prelog Rules say about multiple bonds when they are attached to a chiral carbon?
For multiple bonds, break and add imaginary atoms as follows in the diagram
(Basically you double it, so C=O would equal 2 carbons and 2 oxygens)
>the green line indicates the chiral carbon line
What happens if the priority 4 atom is not at the back?
- If the priority atom 4 is at the front instead, then we are viewing the molecule from the opposite site.
- Therefore, anything that looks clockwise, is really anti-clockwise and vice versa.
- So, you would assign chirality as usual and then reverse the answer.
E.g. in the diagram it looks like S chirality, but H is at the front, so you reverse it meaning it has R chirality.
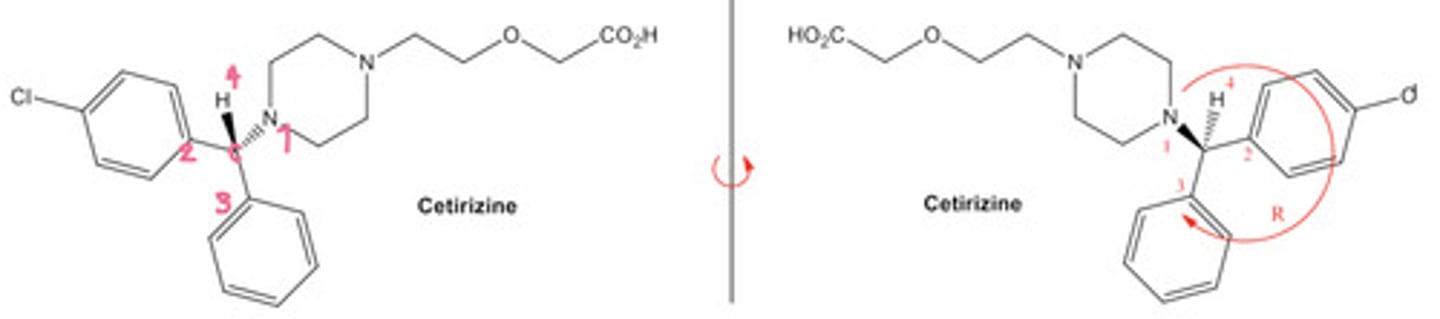
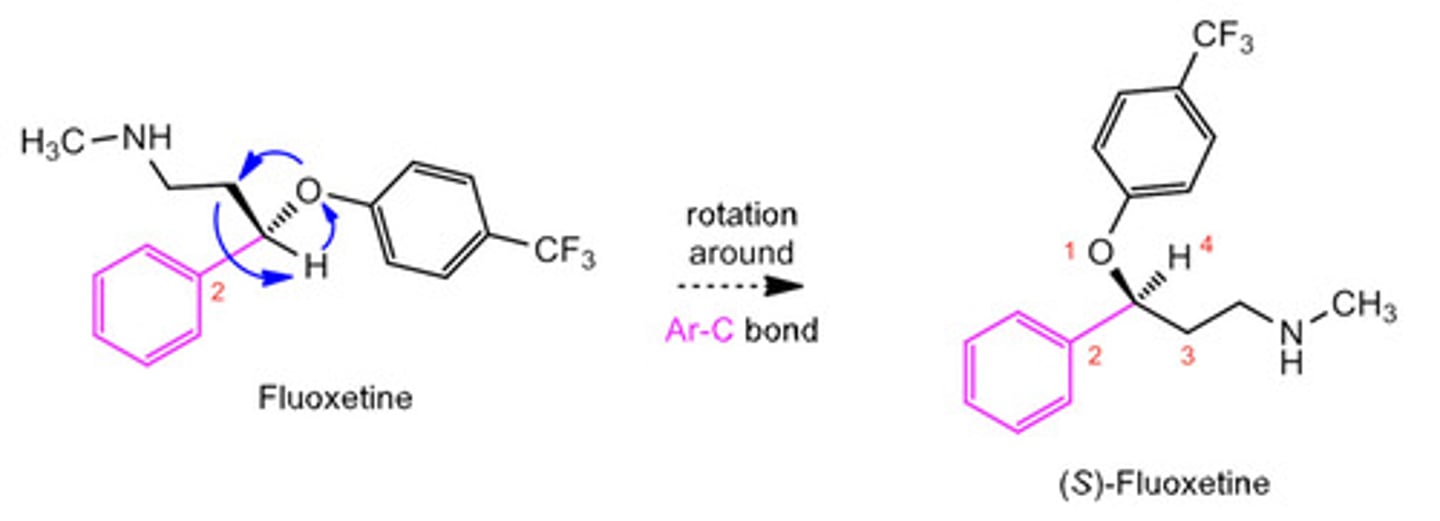
What do we do if the priority 4 atom is not on the dash line or the solid dash at the back? What if its on a single straight line?
We cannot assign the chirality confidently therefore we must rotate the molecule.
- To do this, we keep one bond that is connected to the chiral C fixed/stationary.
- Then, we rotate the rest of the molecule around that bond.
See the diagram
What are the properties of enantiomers?
Similarities and differences between enantiomers
- R and S isomers of a molecule have identical physical and chemical properties (e.g. mp, by, solubility, pka (acidity) etc)
- They have the same functional groups and same skeleton
2 Exceptions:
1. Rotation of plane polarised light (PPL) is different
2. Interaction with other chiral molecules is different
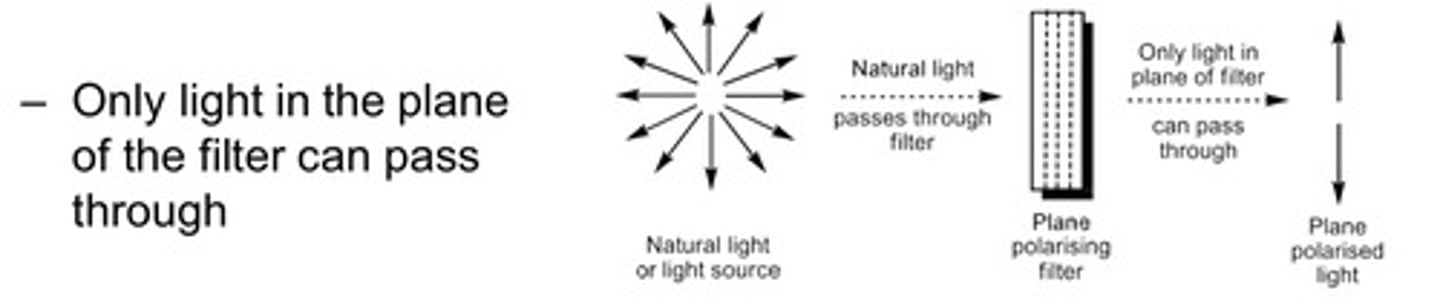
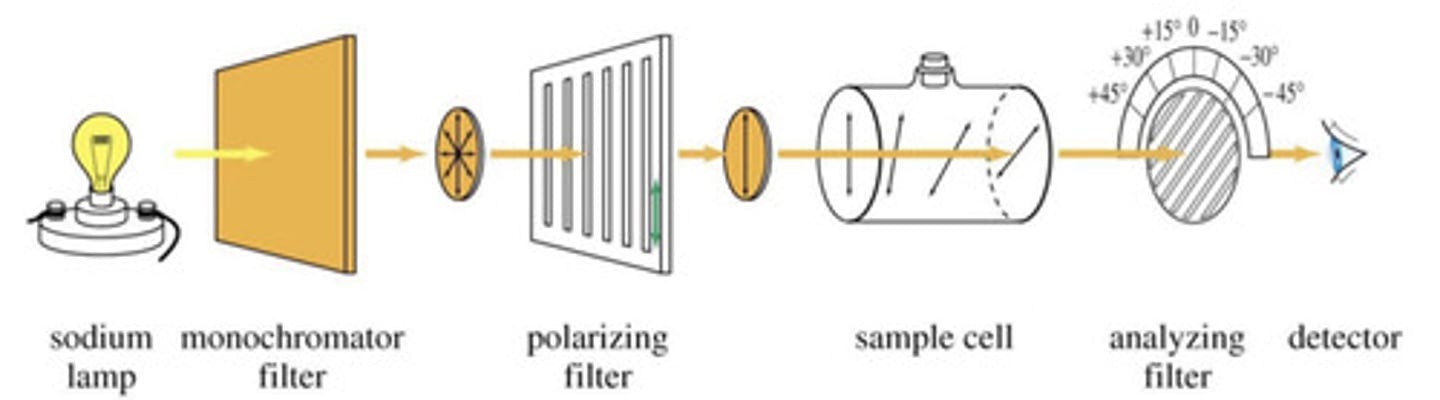
What do we mean by plane polarised light?
- PPL is produced by passing light through a polarising filter.
What is the rotation of PPL measured in?
- Measured in degrees and represented by a[D], using light corresponding to the D line of a sodium lamp.
- The equation allows us to calculate the degree of rotation of the PPL.
![<p>- Measured in degrees and represented by a[D], using light corresponding to the D line of a sodium lamp. <br>- The equation allows us to calculate the degree of rotation of the PPL.</p>](https://knowt-user-attachments.s3.amazonaws.com/bf7ec87f-2cb8-42d8-8dba-a13c1f136968.jpg)
What kind of rotation does each optically active compound have?
- Each optically active compound has a characteristic specific rotation.
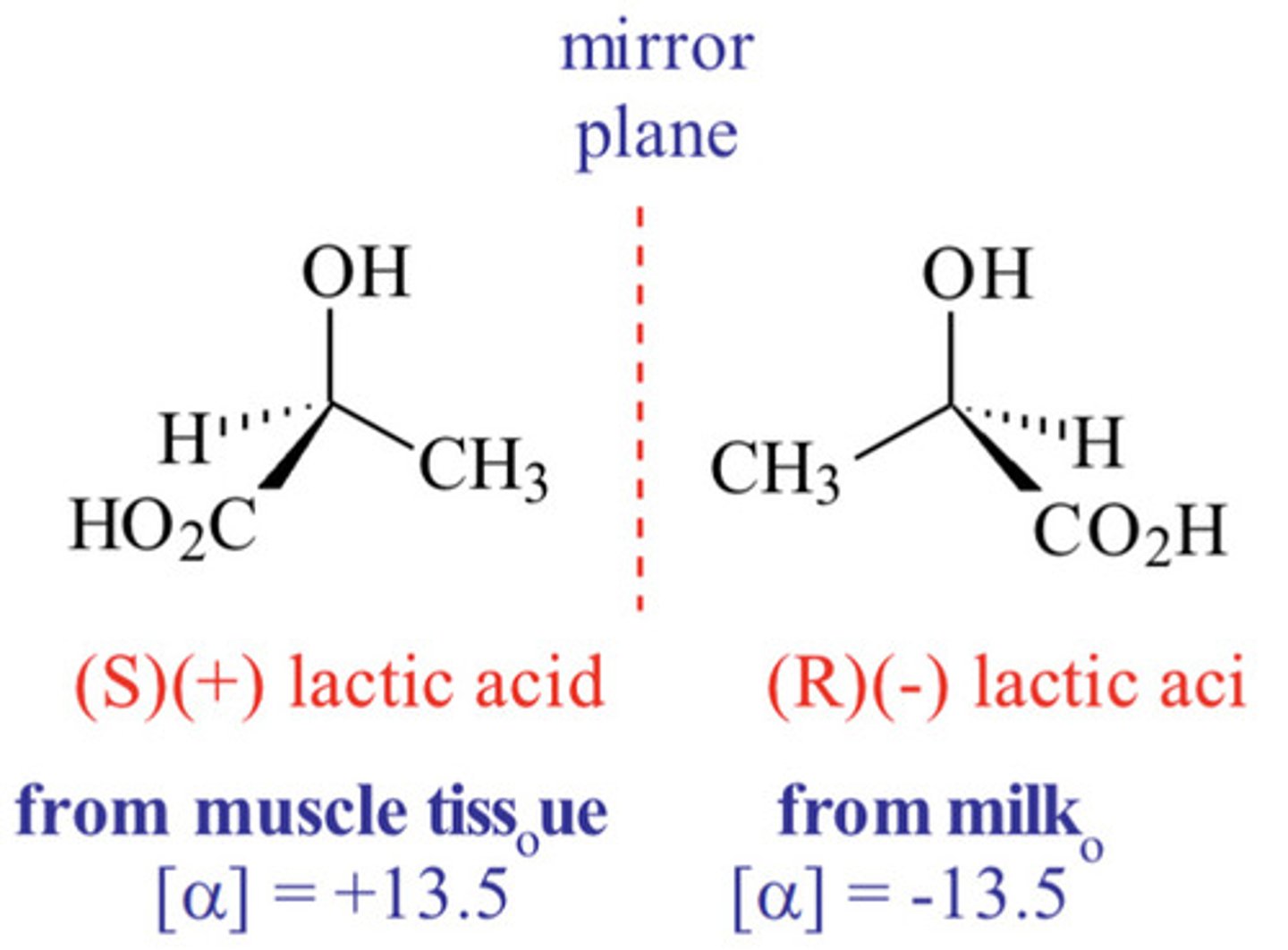
What is a racemic mixture?
A racemic contains an equal amount of the 2 enantiomers, and is optically inactive.
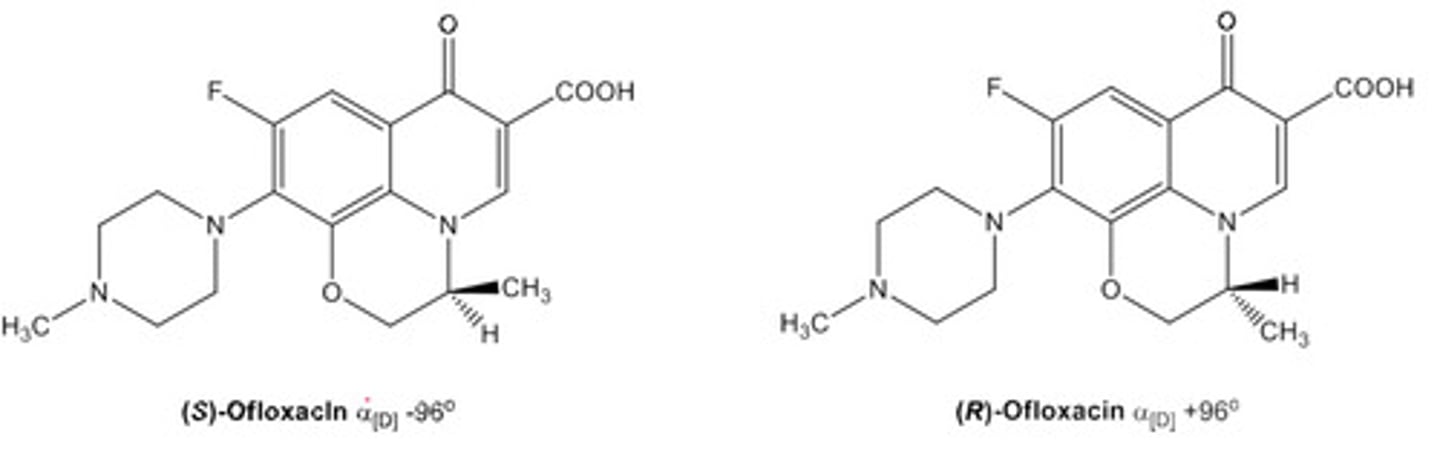
In what directions do enantiomers rotate PPL?
Enantiomers rotate PPL in opposite directions by equal amounts.
- Use '+' for clockwise (d)
- Use '-' for anticlockwise (l)
What is enantiomeric excess?
- Also called ee
- Is the amount of pure enantiomer in excess of the racemic mixture
- If we = 50% then the observed rotation will be only 50% of the rotation of the pure enantiomer.
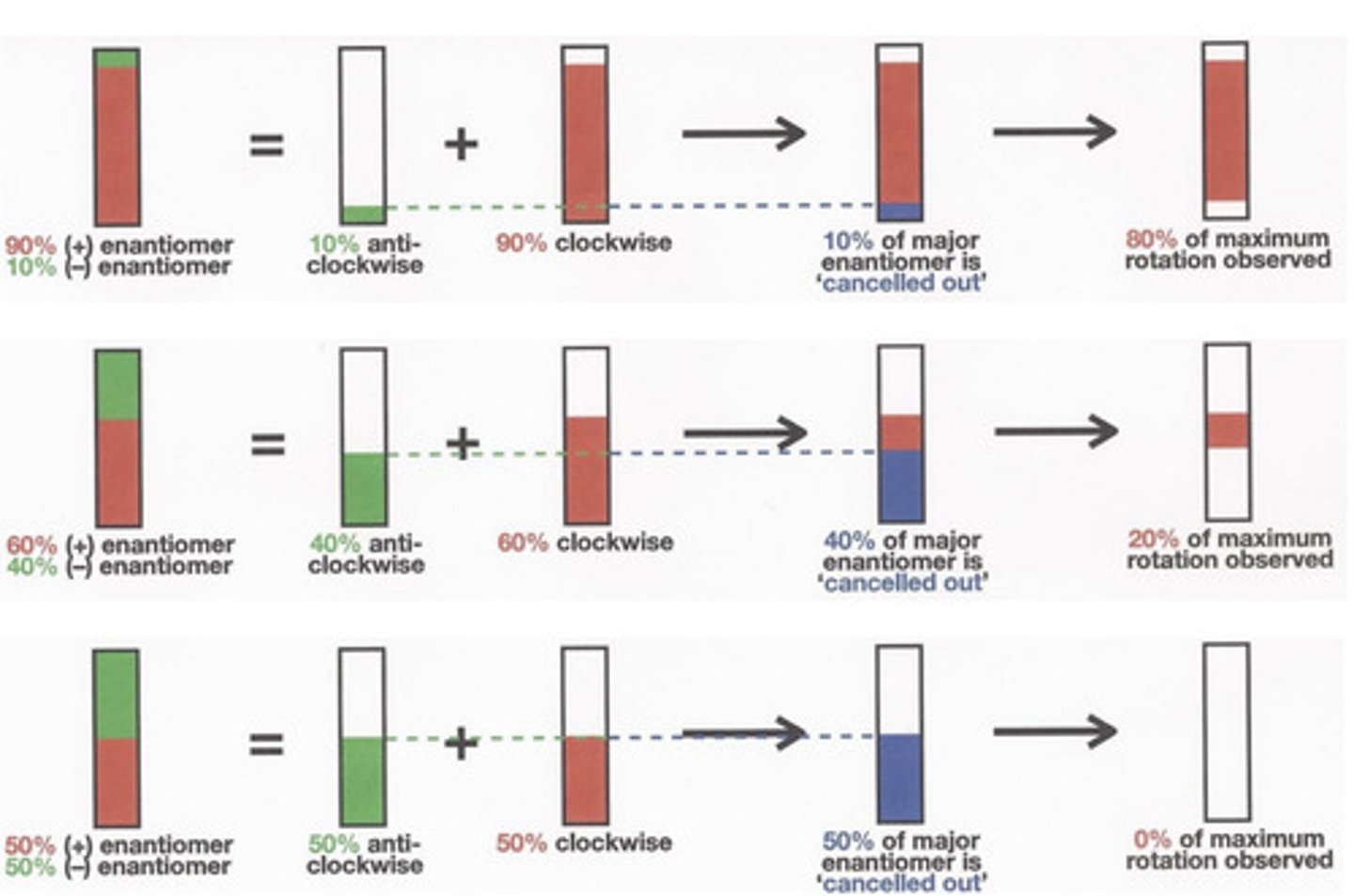
How can we measure enantiomeric excess?
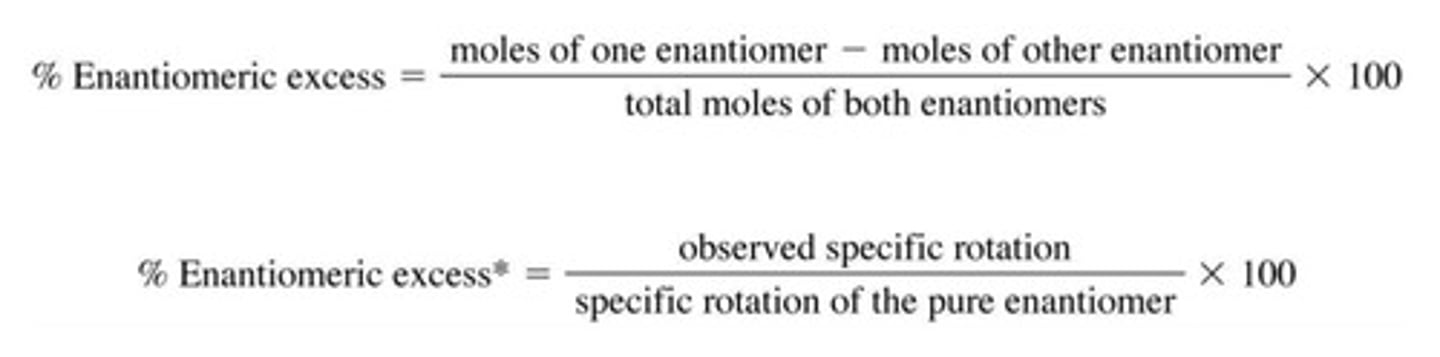
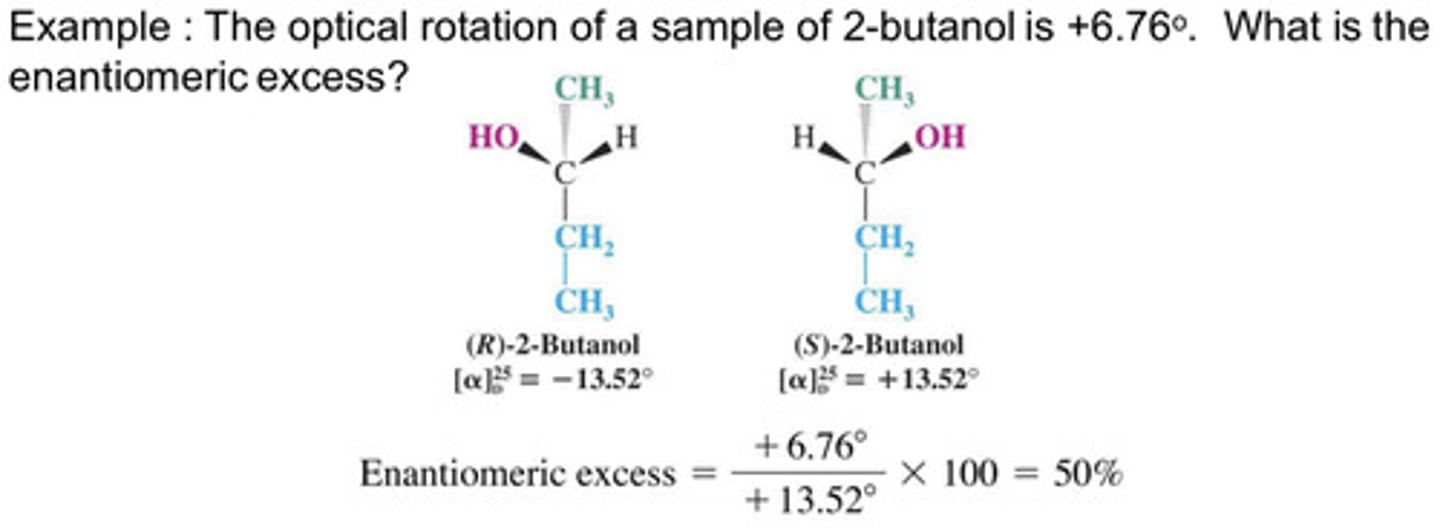
The optical rotation of a sample of 2-butanol is +6.76 degrees. What is the enantiomeric excess?
What is the 3 point model of binding?
(R) enantiomer active binds correctly to receptor to trigger response
(S) enantiomer cannot bind correctly, does not trigger response.
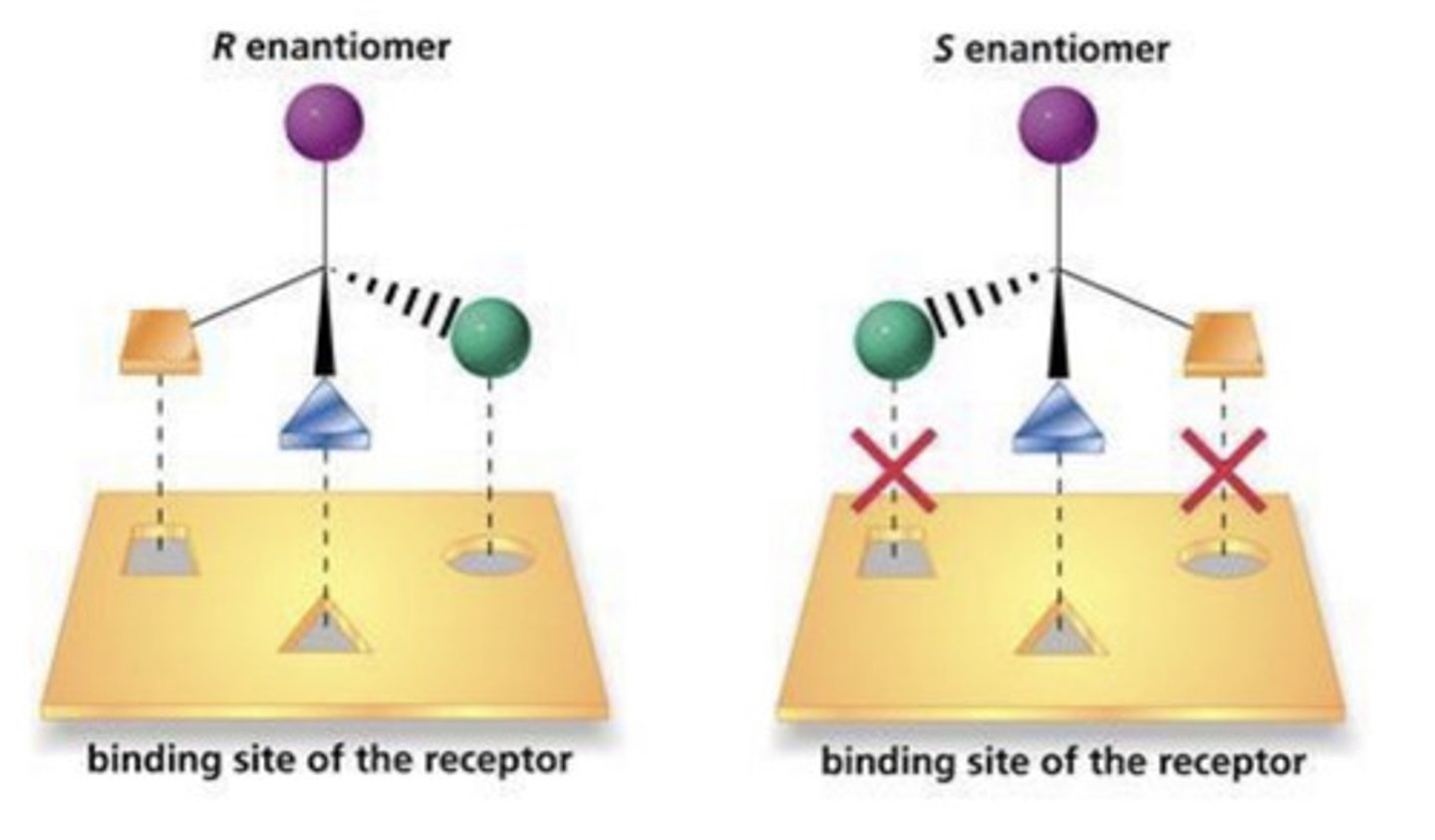
What is often used to explain differences in enantiomer interactions with another chiral specie?
The 3 point model of binding
Why are all enzymes and receptors in the body chiral?
Because they are formed from amino acids which are chiral
What does binding of enantiomers depend on?
Binding depends on 3D shape
- Enantiomers of biological molecules bind with different affinity due to 3D fit
- Enantiomers of pharmaceuticals bind with different affinity due to 3D fit.