GPCR 2: G proteins
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
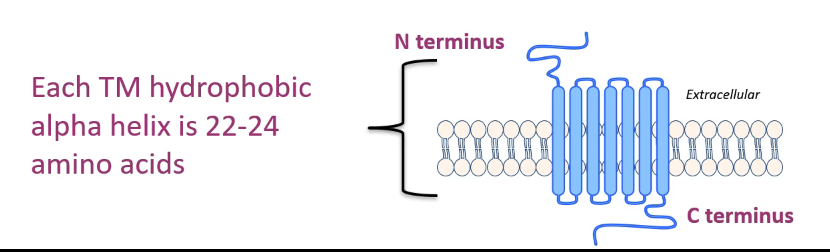
7 transmembrane (TM) domain receptors
7 TM receptors - single polypeptide chain traverses plasma membrane 7 times
3 intracellular & 3 extracellular loops
N terminus = extracellular
C terminus = intracellular
each TM hydrophobic alpha helix = 22-24 AA
important differences: variety of ligands
growth factors, glucagon, epinephrine, prostaglandins, acetylcholine (muscarinic effects), opioid peptides, etc.
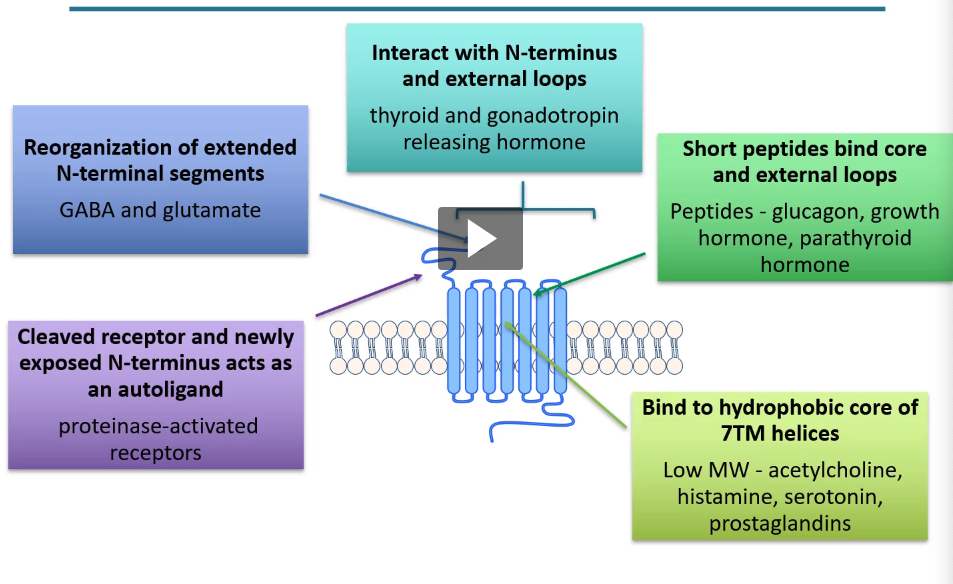
types of ligand-GPCR interactions
binds to hydrophobic core of 7TM helices
low MW - acetylcholine, histamine, serotonin, prostaglandins
short peptides bind core and external loops
peptides - glucagon, growth hormone, parathyroid hormone
interact with N-terminus and external loops
thyroid and gonadotropin releasing hormone
reorganization of extended N-terminal segments
GABA and glutamate
cleaved receptor and newly exposed N-terminus acts as autoligand
proteinase-activated receptors
GPCRs
GPCRs are the targets of 50% of marketed drugs
involved in vision, olfaction, CNS, immune system, digestive system and many other processes
many examples
beta adrenergic receptors
thyrotropin receptors
glucagon receptors
some types of dopamine and serotonin receptors
nucleotides
CTP = important in phospholipid biosynthesis
UTP = polysaccharide assembly
ATP = metabolism and cell activity
GTP = receptor signaling acting as cofactor
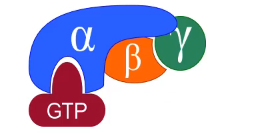
G-proteins
guanine nucleotide-binding proteins
intermediary proteins in signal transduction
found alone inner surface of the plasma membrane
associated with GPCRs
other names = molecular switches and effectors
switches between inactive form (bound to guanine diphosphate, GDP) to active form (bound to guanine triphosphate, GTP)
heterotrimeric proteins (3 subunits)
alpha subunit has the nucleotide binding site
beta and gamma subunits behave as 1 entity
anchored to membrane by lipid attachments
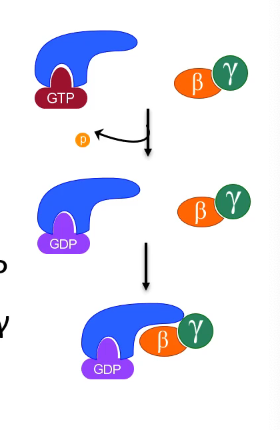
G protein activation
begins as inactive heterotrimer: Gαβγ
agonist binding and receptor conformation change
recruit G protein to agonist-bound receptor
exchange GDP for GTP (on alpha subunit)
the GTP-Gα and Gβγ subunits can go on an activate different signaling cascades through exposed regions
[GTP] > [GDP] = NOT rate limiting step once we have ligand bound to GPCR
![<ol><li><p>begins as <strong>inactive heterotrimer</strong>: G<span>αβγ </span></p></li><li><p><span>agonist binding and receptor conformation change</span></p></li><li><p><span>recruit G protein to agonist-bound receptor</span></p></li><li><p><span>exchange <strong>GDP</strong> for <strong>GTP</strong> (on <strong>alpha</strong> subunit)</span></p></li><li><p><span>the GTP-Gα and Gβγ subunits can go on an activate different signaling cascades through exposed regions</span></p></li></ol><ul><li><p>[GTP] > [GDP] = NOT rate limiting step once we have ligand bound to GPCR</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/74bae22f-59ff-4e40-9fea-7aa35711180d.png)
G-protein inactivation
once Gα subunit comes into contact with effector enzyme, its innate GTPase will be activated
Gα subunits will hydrolyze GTP → GDP
reassociation of Gα with Gβγ
restore inactive form
hydrolysis of GTP is slow as a result, the Gα can interact with number of effector molecules before it returns to GDP bound state → part of amplification of downstream mechanism
alpha subunits
interactions
C-terminus interacts with receptors
N-terminus interacts with βγ subunit
following binding of GTP and detachment of βγ dimer, a surface on α-subunit is revealed for interaction with effector molecule
the diversity of heterotrimeric G proteins = due to alpha subunit
different types of alpha subunits
universal expression (α5, αi, α11, αq)
sensory cells: taste (αt), olfactory (αolf)
neural crest and endocrine tissues (αo)
neurons (αz)
hematopoetic cells (α16)
beta and gamma subunits
dimer subtypes
5 beta subtypes with similar AA identity
12 gamma subtypes with more diverse sequences
identity of the βγ dimer contributes to the coupling of G proteins to particular receptors
the C-terminus of the γ-subunit is modified by geranylgeranyl (20C) or farnesyl (15C) groups to tether the βγ subunit to plasma membrane
functions
ensure localizaiton, coupling and deactivation of α subunit
reduce tendency of GDP to dissociate from α-subunits
regulate affinity of receptors for ligands
regulate receptor phosphorylation by specific kinases
βγ subunits can also act as signaling proteins
increase K+ channel activity
decrease Ca2+ channel activity