Kaarten: MOD1 theme 1 | Quizlet
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
136 Terms
innate immune response
· Dendritic cell/ macrophage -> picks up the pathogen and eats it
· It starts producing cytokines
· Opsonization by the complement system
· Hungry neutrophils and macrophages enter and eat
-> Quick and dirty
-> Fast
-> No memory
-> The same every time
adaptive immune response
· Dendritic cell/ macrophage -> picks up the more challenging pathogen and eats it
· It travels to the lymph node
· It presents the pathogen to a T-helper cell / CD4+ T cell
· The T-helper cell gets activated and helps activate B-cells
· Together they expand and start to produce the specific antibodies
· Once they finish, they serve it to macrophages and neutrophils, that eat
-> This response takes time (2 weeks)
-> More efficient
-> Memory
-> It will be better every time it's used
immunologic reaction to large pathogen (parasite)
· Mast cell picks it up and eats
· Starts producing cytokines to call eosinophil
· The eosinophil releases granules
innate
· Fast
· Less specific (patterns)
· Does not improve
· No memory
adaptive
· Slow
· Highly specific
· Improves overtime
· Memory
inflammation
redness, swelling, heat, pain, which all result in: functional loss = reactions that bring cells and soluble molecules of the immune system to sites of infection or tissue damage
the innate immune system consists of:
· Biochemical barriers
- Skin
- Mucosa
· Soluble proteins
- Anti-microbial peptides
- Complement
- Cytokines/ chemokines
- Acute phase proteins
· Cells
- Granulocytes
- Macrophages
extracellular infection in interstitial spaces/ blood/ lymph
organisms: viruses, bacteria, protozoa, fungi, worms
defense: complement, macrophages, neutrophils
extracellular infection in epithelial surfaces
organisms:
- neisseria gonorrhoeae
- candida albicans
- worms
defense: antimicrobial peptides
intracellular infection in cytoplasm
organisms: viruses, listeria, protozoa
defense: NK cells
intracellular infection (vesicular)
organisms: mycobacteria, trypanosomes, cryptococcus neoformans
defense: activated macrophages
complement system
- Cascade of soluble proteins and cell-bound receptors and inhibitors
- Mainly present in serum
- Produced by a variety of cell types
functions of complement
- Innate immunity: opsonization, lysis of pathogens, chemotaxis, inflammation, cell activation
- Disposal system: clearance of immune complexes and apoptotic cells
- Adaptive immunity: augmentation of antibody response, promotion of t-cell response, elimination of self-reactive B-cells, enhancement of immunologic memory
classical pathway
immune complexes, apoptotic cells -> C1q -> C4b2a -> C3
C3 convertase = C4b + C2a
lectin pathway
carbohydrates, IgA -> MBL -> C4b2a -> C3
alternative pathway
bacterial surfaces, LPS, IgA -> C3H2O -> C3bBb -> C3 (is inhibited by factor H!!)
C3 convertase = C3b + Bb
after C3:
-> C3a
-> C5a
-> C5b-9 (MAC)
-> C3b
convertase
any of several enzymes that convert a compound into smaller, biologically-active compounds
Anaphylatoxins (C3a, C5a) -> activation of inflammation
increase vascular permeability -> more plasma proteins at site of infection + migration and activity of monocytes and neutrophils into tissue
Opsonization
An immune response in which the binding of antibodies to the surface of a microbe facilitates phagocytosis of the the microbe by a macrophage
membrane attack complex (MAC)
complement system components assembled to form pores in membranes of invading cells
5 stages of innate immune response
1 - recognition of infection or damage
2 - recruitment of cells and soluble factors
3 - elimination of microbe
4 - resolution of inflammation
5 - induction of adaptive immunity (if necessary)
recognition
- Distinguish 'self' from 'non-self' or 'healthy' from 'damaged' tissue
- Pattern recognition receptors (PRR) are activated by:
· Pathogen-associated molecular patterns (PAMPs)
· Damage-associated molecular patterns (DAMPs)
acute inflammatory response:
1. Dilatation
2. Increased vessel wall permeability
3. Leukocyte emigration
Redness - swelling - heat - pain - functional loss
Natural killar cells
effector functions
1. Kill directly
2. Source of cytokines (IFN gamma)
3. Antibody dependent cytotoxicity: enhance adaptive immune response
resolution, repair and healing
1. Macrophages
2. Fibroblasts
3. Angiogenesis
antibody dependent cell-mediated cytotoxicity (ADCC)
1. antibody binds antigens on the surface of target cell
2. Fc receptors on NK cell recognize bound antibody
3. Cross-linking of Fc receptors signals the NK cell to kill the target cell
4. target cell dies by apoptosis
Common hematopoietic stem cell
origin of blood and immune cells
characteristics of B and T cells
1. antigen specificity
2. one cell - one specificity
3. B cell host defense extracellular pathogens
4. T cell host defense against intracellular pathogens
clonal selection
The process by which an antigen selectively binds to and activates only those lymphocytes bearing receptors specific for the antigen.
somatic recombination
The process by which DNA segments are rearranged to form a variable region for either the B cell or T cell receptor
alternative splicing
Splicing of introns in a pre-mRNA that occurs in different ways, leading to different mRNAs that code for different proteins or protein isoforms. Increases the diversity of proteins.
phases of B cell development
1. repertoire assembly
2. negative selection
3. posisitve selection
4. searching for infection
5. finding infection
6. attacking infection
Repertoire assembly
Generation of diverse and clonally expressed B-cell receptors in the bone marrow
negative selection
Alteration, elimination or inactivation of B-cell receptors that bind to components of the human body
positive selection
Promotion of a fraction of immature B cells to become mature B cells in the secondary lymphoid tissues
Searching for infection
Recirculation of mature B cells between lymph, blood, and secondary lymphoid tissues
Finding infection
Activation and clonal expansion of B cells by pathogen-derived antigens in secondary lymphoid tissues
Attacking infection
Differentiation to antibody-secreting plasma cells and memory B cells in secondary lymphoid tissue
TRC
- cannot be secreted, because it is bound to the membrane
- is generated by recombination
- = antigen specific
- T-cell receptors recognize peptide antigen presented in MHC molecules
- cannot directly recognize an antigen
Allotype
Antigenic differences within a given class of Ig between members of the same species
T cell precursors
travel from the bone marrow to develop in the thymus -> mature T cells leave the thymus and travel to secondary lymphoid tissues
Germinal center reaction
Production of B cells producing high-affinity isotype-switched antibodies and memory B cells.
affinity maturation
The increase in affinity of the antigen-binding sites of antibodies for the antigen that occurs during the course of an adaptive immune response. (b cells only!)
MHC class I (expression)
all nucleated cells (so no erythrocytes)
-> intracellular antigens
-> activates CD8+ cytotoxic t cells -> killing
MHC class II (expression)
B cells, macrophages and dendritic cells = professional antigen presenting cells (APC)
Different origin
-> extracellular antigens
-> CD4+ -> T helper cells that activate B cells to make antibodies
T cell activation
· Naïve T cells bind transiently to the antigen presenting cells they meet
- TCR screens peptide: MHC complexes
· Delivery of antigen-specific signal through the TCR
- T cell-APC interaction is stabilized
· Second signal is required to trigger naïve T cell activation:
- Role of co-stimulation
* leads to IL-2 driven T cell proliferation
Co-stimulation
Complete T cell activation requires T cell to also bind to one or more co-stimulatory signals on surface of APC. CD28 (T cell) -B7 (APC)
cyclosporin, tarcolimus and rapamycin (immunosurpressive drugs)
supress IL-2 action/ production
B cell activation
· Crosslinking of B cell receptor: antigen-specific signal
· Additional signals are required for B cell activation:
- B cell co-receptor complex:
Ø Surface Ig (specificity)
Ø Ig alfa and Ig beta (signaling)
Ø CD 19 (signaling chain)
Ø CD21 (CR2 complement)
Ø ...
T cell independent antigens
do not require help of T cells for activation of B cells, however:
· No induction of B cell memory
· No class switching (only IgM)
Examples: bacterial polysaccharides with repeating epitopes
Importance: problem for vaccine development
T cell dependent antigens
(proteins) that evoke B cell responses are T cell dependent (requiring CD4 cells)!
· Induction of B cell memory
· Affinity maturation
· Class switching
Tfh cells
Help B cells become activated, switch isotype, and increase antibody affinity
somatic hypermutation
mutation that occurs at high frequency in the rearranged variable-region DNA segments of immunoglobulin genes in activated B cells, resulting in the production of variant antibodies, some of which have a higher affinity for the antigen
Isotype switching
DNA recombination of the constant part of the Ig molecule, while maintaining the same Fab fragment (so the same specificity)
eg: IL-4 / IL-13 switch to IgE
IL-5 activation and expansion eosinophils, basophils
Tolerance
regulation of unwanted B and T cell immunity
Central tolerance
· Selection during maturation in the bone marrow (B cells) or thymus (T cells)
- Immature B cell in bone marrow: binding to self-antigen can lead to deletion or inactivation of immature B cells
- T cells specific for and binding too strongly to self-antigens are removed in the thymus by negative selection
peripheral tolerance
· Regulation of B and T cell responses after the cells have left the primary lymphoid organs: anergy, regulatory T cells
Anergy
the lack of responsiveness to an antigen despite the presence of antigen-specific lymphocytes
B cell receptor
Membrane bound immunoglobulins serve as B cell receptor
· B cells are characterized by the presence of surface immunoglobulin: the B cell receptor (BCR)
· Each B lymphocyte has multiple copies of the same B cell receptor
· Each B cell generates BCR's with a single specificity
· The repertoire of BCRs is capable of recognizing millions of antigens
B cell stage: resting B cell
* surface Ig
* Surface MHC class II
* growth
* somatic hypermutation
* isotype switching
B cell stage: plasma stage
* high rate Ig secretion
Fab
fragment antigen binding
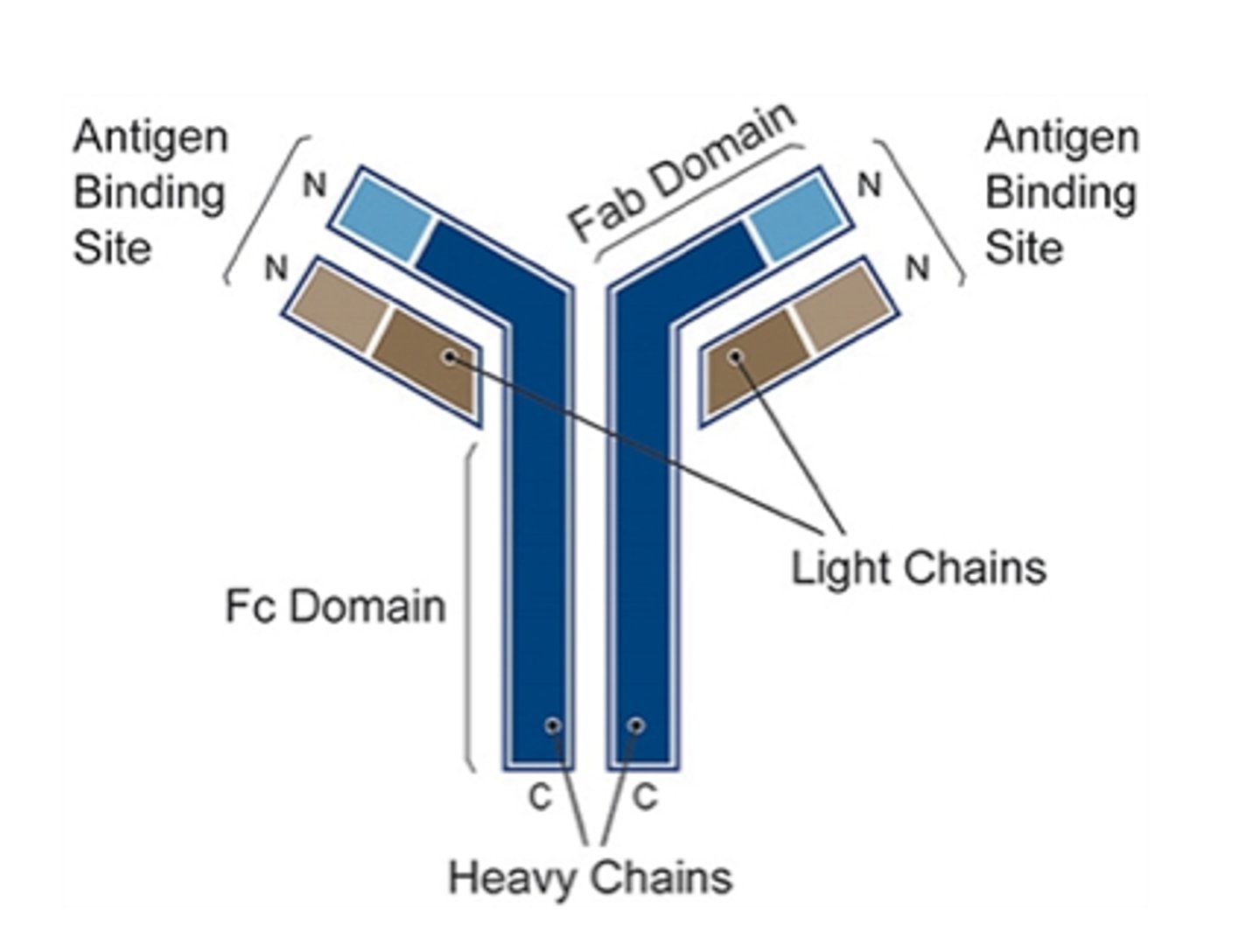
Fc
fragment crystallizable
Antibody functions
neutralization, opsonization, complement activation
Complement-dependent cytotoxicity
IgG molecules bind to antigens on bacterial surface -> C1q binds to two or more IgG molecules and initiates complement activation (also binds to antigen-bound IgM)
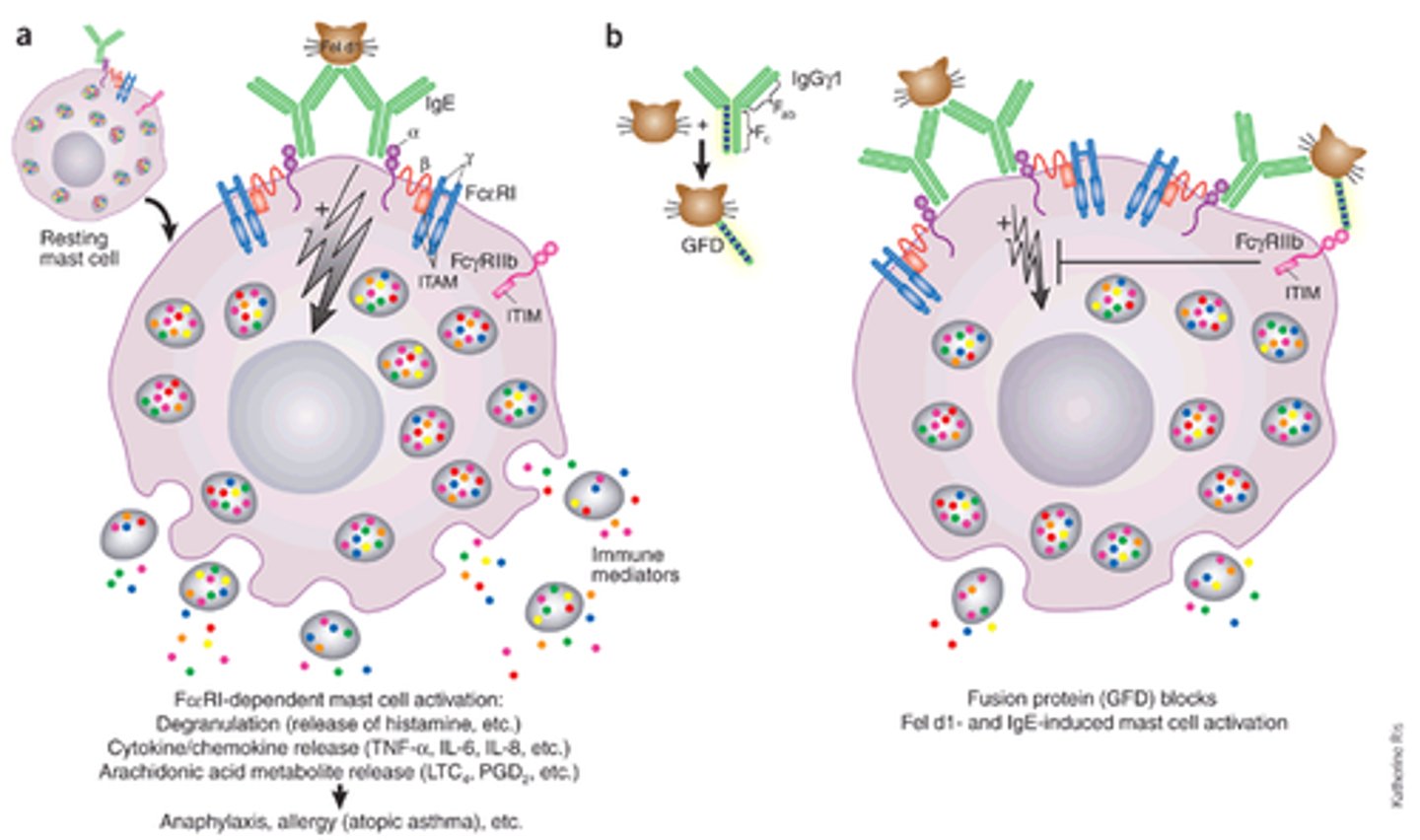
mast cell degranulation
Multivalent antigen cross-links with IgE on cell surface, causing release of granule contents
ABO-system
IgM
presense of antibodies during development
IgG: before birth - 9 months, than upwards
IgM: -3 - 1 year (max)
IgA: from 3 months
monoclonal antibodies
a collection of identical antibodies that interact with a single antigen site
polyclonal antibodies
Antibodies produced by injecting animals with a specific antigen. A series of antibodies are produced responding to a variety of different sites on the antigen.
acute cardiac tamponade
build-up of fluid, blood or air in the pericardial sac
hypertrophy
increase in size of cells, resulting in an increase in the size of an organ
- Physiological = functional demand
- Pathological = disease processes (e.g. left ventricle hypertrophy)
Normal cardiomyocytes -> stress -> cardiac myocytes ^synthesis of myofilaments -> bigger = hypertrophy
atrophy
reduce in cell size or cell number, which may result in an decrease in the size of the organ
- Physiological (eg. Thymus)
- Pathological (eg. Cachexia
causes of atrophy
· Decreased workload (muscle)
· Denervation
· Decreased blood flow
· Decreased nutrition
· Aging (involution - endocrine glands)
· Pressure (tumor)
Normal myocytes -> long-standing disuse -> loss of muscle mass
examples of atrophy
Generalized cerebral atrophy occurs naturally during aging and may be referred to as physiological
Several injuries or diseases however can cause pathological cerebral atrophy (stroke, trauma, Alzheimer's etc.)
hyperplasia
= increase in number of cells, resulting in an increase in size of the organ
è Only in organs with stem cells that can differentiate and mature (e.g. Liver)
è Organ adapts to stress
è Compensatory hyperplasia = in organs that regenerate -> liver, skin, lining of intestines, bone marrow
è Hormonal hyperplasia = in organs regulated by hormones -> endocrines / reproductive system
è Physiological = enlargement of breasts during pregnancy
è Pathological = excessive hormonal stimulation
Metaplasia
a substitution of one differentiated cell or tissue types, for another type of cell
- E.g. columnar to squamous (or other way)
- Often at a junction between inside and outside world:
> Gastro-esophageal
> Recto-anal
> Ecto-endocervix
Cellular adaptation in bronchial epithelial lining after smoking = squamous metaplasia
Barrett's disease
esophagus lining changes from squamous to columnar because of reflux
Causes of metaplasia
· Hypoxia (decrease O2)
· Physical agents (e.g. trauma)
· Chemical agents (medication, alcohol)
· Infectious agents (viral)
· Immunologic (auto-immune disease)
· Nutritional (vitamin deficiency)
- Reduces oxidative phosphorylation
- ATP depletion
- Cellular swelling
irreversible changes to cells
· Mitochondrial irreversibility
· Irreversible membrane defects
· Lysosomal digestion
no nuclei, granulocytes attracted to the dying cells
Apoptosis
a pathway of cell death that is induced by a tightly regulated suicide program
· Physiologic (pre-programmed)
> Fetal development
> Replacement skin/ mucosa
· Pathologic
> Organ rejection
> Some viral infections (hepatitis)
· Intrinsic pathway (mitochondrial pathway)
· Extrinsic pathway (death receptor pathway)
Apoptosis: morphology
· Decrease in cell size (shrinkage)
· Increase in chromatin concentration (hyperchromasia, pyknosis -> karyorhexis -> karyolysis)
· Increase in membrane 'blebs'
· End result = phagocytosis
apoptic bodies
Pockets or sacs of a cell that break off when a cell is undergoing apoptosis. Eventually the whole cell breaks into these, and they are then eaten by other cells.
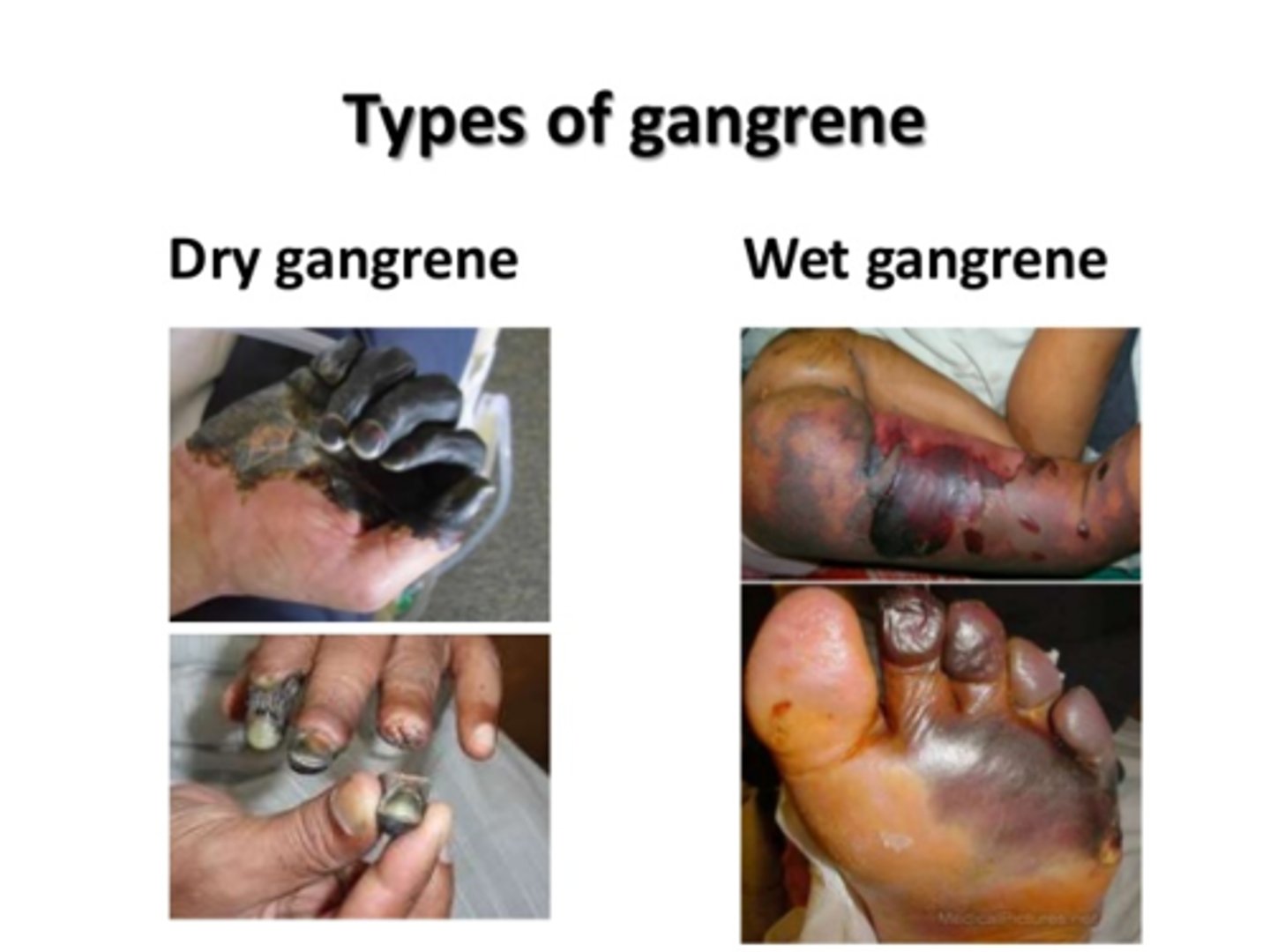
Coagulative necrosis
loss of nucleus but cell shape and organ structures are preserved by coagulation of proteins. In other words, necrotic tissue that remains firm.
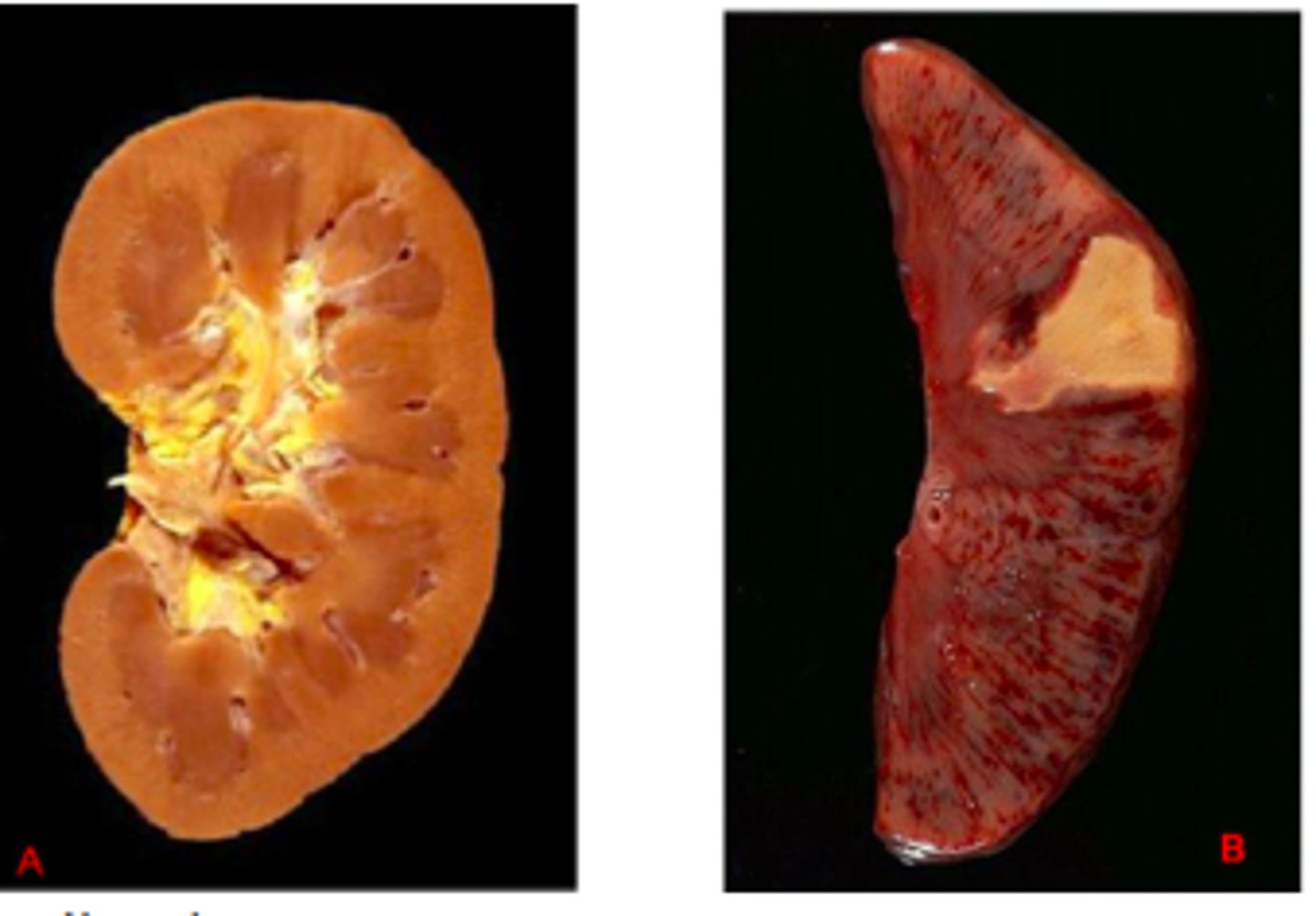
Gangrenous necrosis
Coagulative necrosis that resembles mummified tissue
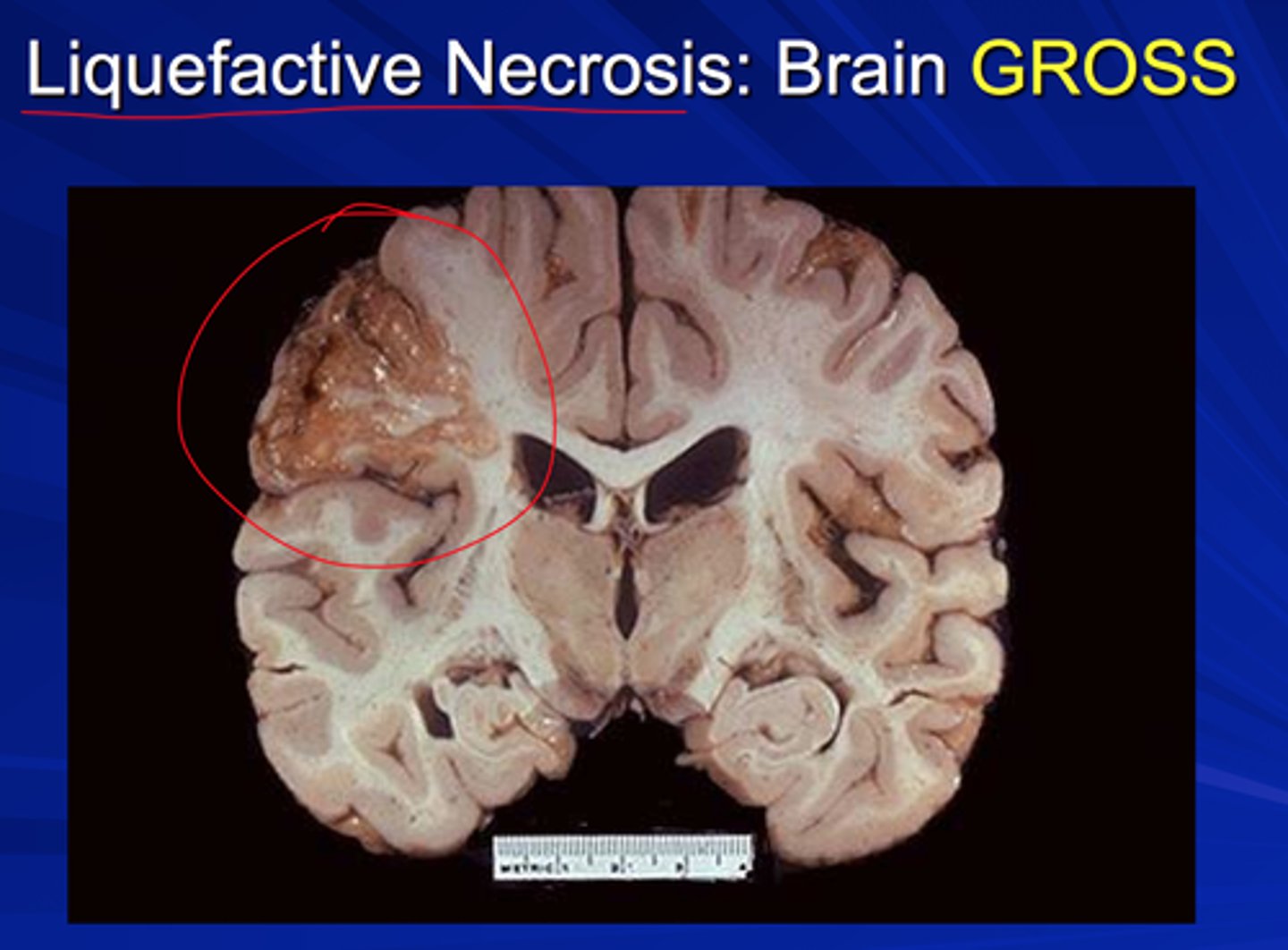
liquefactive necrosis
commonly results from ischemic injury to nerve and glial cells in brain; injured cells release hydrolases that digests brain tissue; tissue becomes soft, liquefies, and segregates, forming cysts. May be caused by staph, strep, or E. coli infections.
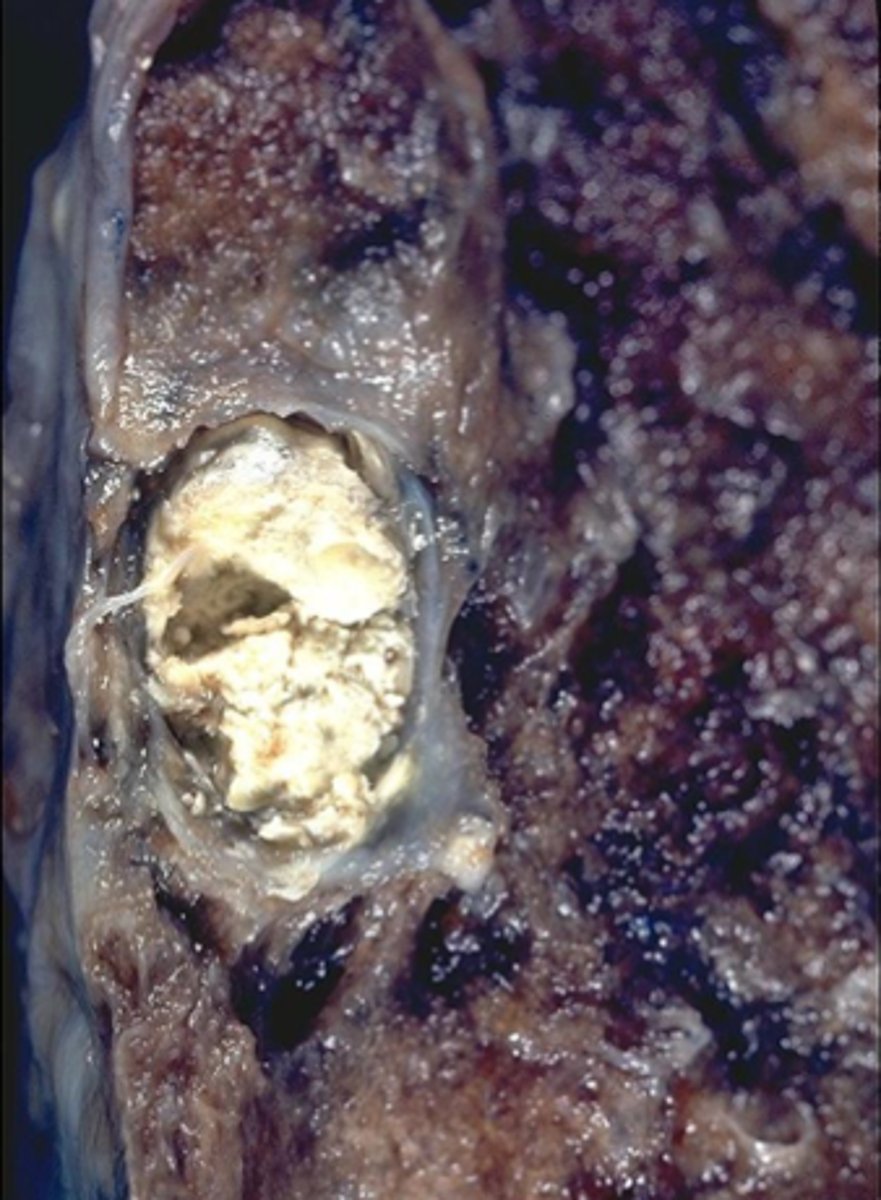
caseous necrosis
degeneration and death of tissue with a cheese-like appearance
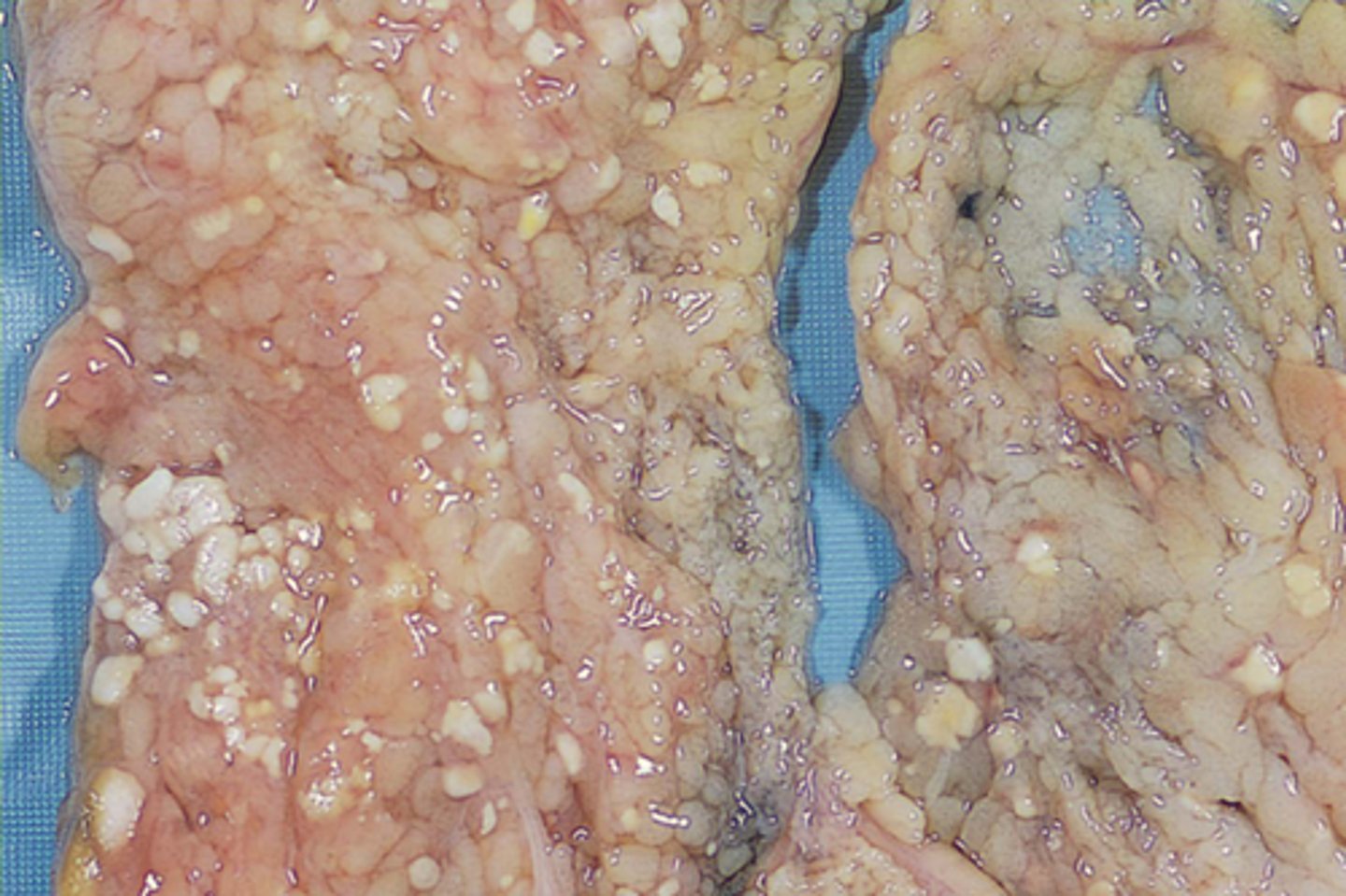
Fat necrosis
Fatty tissue is broken down into fatty acids
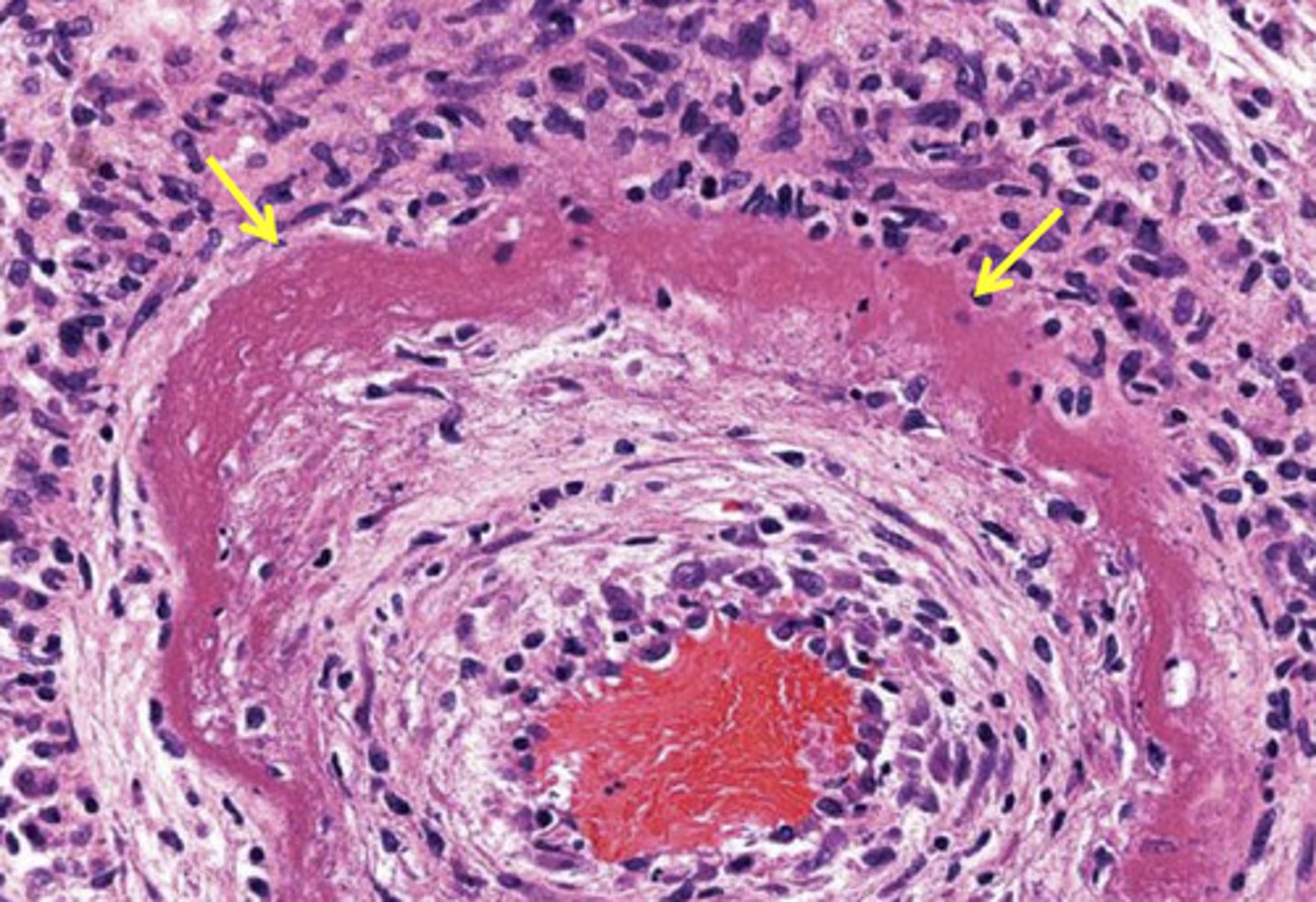
Fibrinoid necrosis
usually blood vessels
death of a cell is replaced by fiber
microscopy: necrosis
- enlarged
- karyolysis
- disrupted plasma membrane
- enzymatic digestion of cellular content
- adjacent inflammation
- invariably pathologic
microscopie: apoptosis
- reduced
- fragmentation
- intact plasma membrane
- possible intact cellular content
- no adjacent inflammation
- often physiologic
Hypoxia
Low oxygen saturation of the body, not enough oxygen in the blood
ischemia
an inadequate blood supply (oxygen and nutrients) to an organ or part of the body, especially the heart muscles.
abscess
incapsulated infection
Signs of inflammation
redness, heat, swelling, pain, loss of function
inflammation: pathoglogy
is a reaction of a vascularized tissue to a pathogenic insult. It is characterized by the generation of inflammatory mediators and movement of fluid & leukocytes from the blood in to the tissue
neutrophil
A type of white blood cell that engulfs invading microbes and contributes to the nonspecific defenses of the body against disease.
multi lobulated nucleus
reasons for inflammation
* infection
* immune reactions
* tissue necrosis
* foreign bodies
changes blood vessels during inflammation
Nitric oxide (NO) and histamine -> histamine, bradykinin, leukotrienes
Vasodilation -> increased permeability -> slower flow -> stasis