Required Practical 1: Making soluble salts
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Salts (e.g. Copper sulfate) positive ion comes from…
metal ion
Which chemicals give metal ion? With an example
metal - copper
metal oxide - copper oxide
metal hydroxide - copper hydroxide
metal carbonate - copper carbonate
Salts negative ions comes from…
acid
Which chemicals give negative ion?
acid, e.g. sulfuric acid
Equiment needed to make soluble salt?
Process of making soluble salt p1
Start with a fixed volume of dilute sulfuric acid (limiting reactant - used up)
to ensure no acid remain at the end as it will contaminate salt
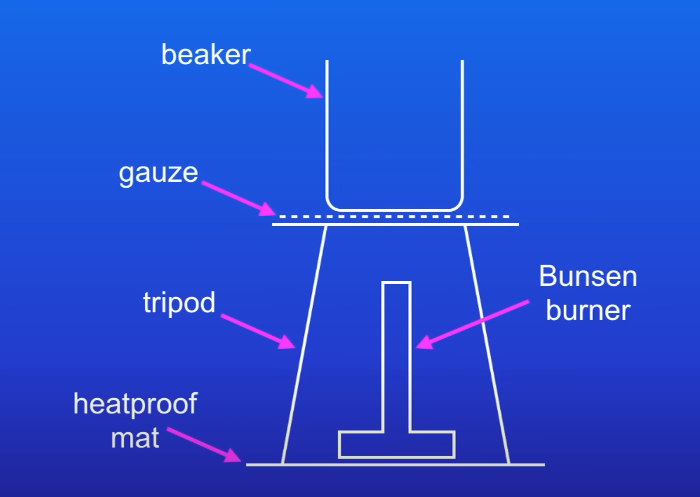
Gently heat acid until almost boiling
could potentially boil over when adding other reactants and that’s dangerous
Use spatula to add small amounts of copper oxide to acid
Stir solution with glass rod
Copper oxide will react and seem to disappear forming a blue colour
Continue adding copper oxide if solution continues to be clear blue
Stop adding coper oxide if some powder remains after stirring
Reaction is complete - all of acid has reacted
Process of making soluble salt p2
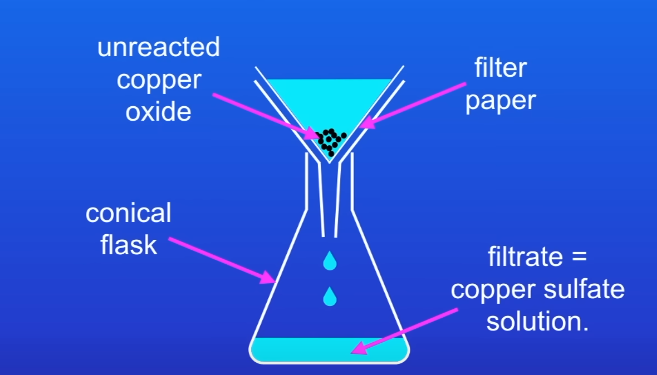
Use flitration to remove unreacted copper oxide
Place solution in evaporating basin
Heat gently over a beaker of boiling water
Heat until half of solution remains
Leave solution for 24 hours in a cool place for crystals to form
Scrape crystals onto paper towel and gently pat them dry