Anticancer - cytotoxic drugs (chemotherapy)
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
22 Terms
Cytotoxic drugs (chemotherapy)
If patients don’t have an obvious driving mutation must treat with cytotoxic drugs
Chemotherapies try to kill fast-growing cells
Drugs target different phases of the cell cycle – mainly DNA synthesis or replication or segregation (S phase) of genetic material (M phase)
However, cancer cells develop efflux mechanisms or increase the repair system to avoid this
The cell cycle
G1 – prepares cells for DNA synthesis
Making proteins
Cell grows larger
S phase – cell generates complete copy of genetic material
2 copies of DNA make in preparation for dividing
G2 phase – cell prepares for mitosis
Cell continues to grow and make more proteins for the final stage
M phase – replicated DNA is condensed and segregated into chromosomes
Cell splits and evenly distributes the DNA between the two cells
G0 phase – resting state (some cells establish mechanisms to stay in G0 phase until the drug is removed)
Throughout the cell cycle cells have checkpoints to ensure that mutated cells are not allowed to replicate
Chemotherapy drugs
Some can act on cells even during the resting phase whilst some can only act at specific cell cycle stages
E.g: some bind to the DNA during replication preventing the DNA from being replicated
E.g: some prevent the cells from splitting into daughter cells by preventing the DNA from being equally divided between the cells
Also damage healthy cells which divide rapidly —> hair cells, GI mucosa cells, bone marrow cells etc. which are responsible for causing side effects.
Combination chemotherapy can be used to target different phases of the cell cycle
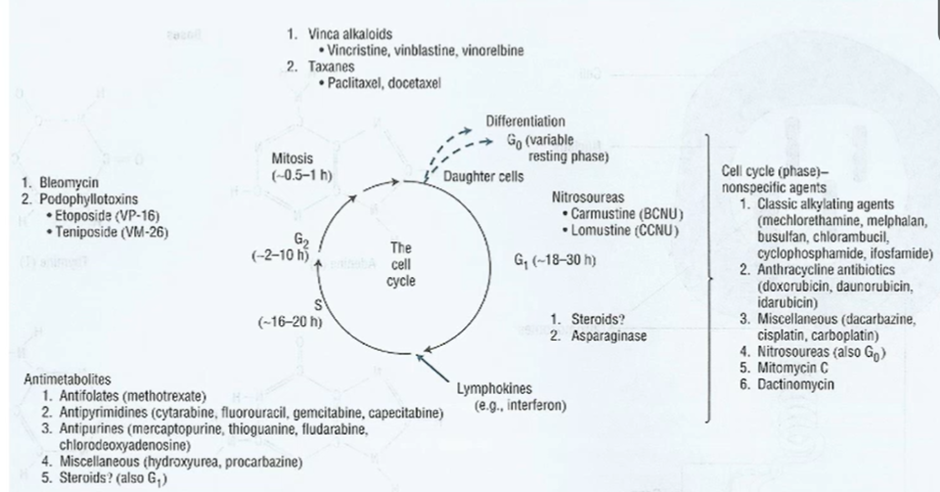
Classes of chemotherapy
Generally, all classes of chemotherapy directly interact with the DNA or prevent the segregation of DNA
Alkylating agents: interact with DNA to stop DNA synthesis
cross link DNA so it cant form 2 strands
mainly affects S phase but also G1
Taxanes: prevent segregation by targeting the microtubules
Topoisomerase 2 inhibitors: interacts with DNA synthesis by preventing the unwinding of supercoiled DNA
Platinum complexes
cisplatin reduces DNA so it cant separate
Anthracyclins: also interact with DNA
Antimetabolites: prevent DNA synthesis
Tubulin interactive agents: prevent M phase occurring

Antimetabolites
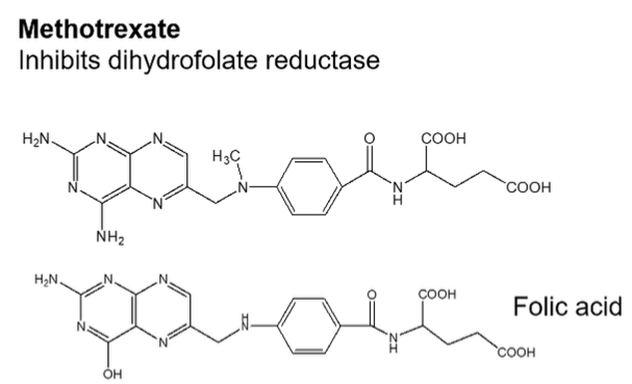
Methotrexate
5-FU
Cytarabine
Methotrexate
Methotrexate is an analogue of folic acid —> which inhibits dihydrofolate reductase and interferes with folic acid production which is required for nucleic acid synthesis
Selective rescue of normal cells may be achieved with leucovorin (citrovorum factor)
Ways that the cancer can confer resistance:
Decreased transport
Decreased affinity of target enzyme
Gene amplification and increased synthesis of target enzyme
Antimetabolites
Methotrexate
5-FU
Cytarabine
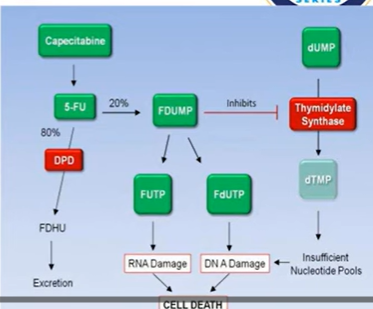
5-FU (5 fluorouracil)
5-FU analog of thymine (crucial nucleotide of DNA replication)
Metabolised into ribo and deoxyribonucleoside phosphates
Inhibition of thymidylate synthetase by 5-fluoro-2-deoxyuridide-5-monophosphate
In addition, theres incorporation of 5-fluorouridide triphosphate into RNA
Resistance cofered by:
Increased synthesis of altered affinity target enzymes
Decreased activation
Increased catabolism
Works throughout the cell cycle, mainly S phase
Toxicity: myelosuppression, nausea, vomiting, anorexia, alopecia
Therapeutic use:
GI tract adenocarcinomas
combination protocols for breast cancer
Topical application for premalignant keratoses
Antimetabolites
Methotrexate
5-FU
Cytarabine
Cytarabine
Cytarabine is an analogue of pyrimidine nucleoside but with the substitution of arabinose for ribose
Chemical nature: 1-beta-arabinofuranosylcytosine
The triphosphate metabolite inhibits DNA polymerase
Resistance is conferred by:
Decreased kinase activity required for activation
Increased inactivation by deaminase
Cell cyle specific for the S phase and blocks progression from G1 to S phase
Causes nausea, vomiting, myleosupression
Excreted chiefly as the noncytotoxic metabolite uracil arabinoside
Deamination can be inhibited by tetrahydrouridine
Plant alkaloids - mitotic inhibitors
Vinca alkaloids: vincristine, vinblastine and vinorelbine
Taxanes: paclitaxel and docetaxel
Podophyllotoxins: etoposide and tenisopide
Camptothecan analgoues: irinotecan and topotecan
Vinca alkaloids
Vinca alkaloids:
Vinblastine: (velban)
Vinblastine sulfate is the salt of a dimeric alkaloid from the plant vinca rosa
Binds to tubulin and interferes with spindle assembly
Resistance conferred by decreased cellular uptake or increased efflux
Specific for mitosis but at high concentrations inhibits S and G1
Causes nausea and vomiting and leukopenia
Vincristine: (oncovin)
Vincristine sulfate is the salt of a dimeric alkaloid from the plant vinca rosa.
Differs from vinblastine in the substitution of an aldehyde for a methyl group
Binds to tubulin and interferes with spindle assembly in mitosis
Resistance conferred by decreased cellular uptake or increased efflux
Cause numbness and tingling of fingers and toes, hair thinning and minimal myelosuppression
Taxol
Antimicrotubule agent which inhibits the microtubule structures within the cell
Blocks in late G2/M
Intercalators
Work by inhibiting topoisomerase
Generate stable ternary complex between the DNA, enzyme and the drug – with the DNA strands cleaved so causes starnd cleavage which is less well repaired by cancer cells
Type 1 topoisomerase: campothecin
Type 2 topoisomerase: etoposide
Type 2 topoisomerase overexpressed in some resistance
Podophyllotoxins- Etoposide:
Semi synthetic alkaloid derived from podophyllotoxin
Binds to tubulin but this is not believed to be important for thereputic effect —> may stimulate topoisomerase 2 to cleave DNA
Greatest lethality in S and G2 phase
Causes leukopenia, alopecia, nausea and vomiting more common with oral administration
Antibiotics: dactinomycin
An antibiotic from a streptomyces species
Contains two cyclic peptides which are linked by a chromospore moiety
Binds noncovalently to DNA
Intercalated between adjacent GC base pairs
Inhibits RNA polymerase more than DNA polymerase
Resistance conferred by decreased ability of cells to take up or retain the drug
Cell cyle stage non specific
Causes nausea, vomiting, local vesicat, myelosuppression, redness of skin where radiation has been given, alopecia
Antiobiotc drugs:
Daunorubicin (daunomycin, rubidomycin)
Doxorubicin (Adriamycin)
Bleomycin (blenoxane)
Daunorubicin
An anthracycline glycoside isolated from streptomyces spp
Red colour
Intercalated between base pairs of DNA and inhibits RNA synthesis
Resistance conferred by decreased uptake or more rapid removal or the drug
Non specific cell cycle stage
Causes nausea, vomiting, myelosuppression, cardiomyopathy, alopecia’
Antiobiotc drugs:
Daunorubicin (daunomycin, rubidomycin)
Doxorubicin (Adriamycin)
Bleomycin (blenoxane)
Doxirubicin
Same chemical nature as daunorubicin except theres an additional hydroxyl group
Same mechanism and side effects as daunorubicin
Therapeutic use for leukemias, lymphomas, solid tumours including sarcomas
Antiobiotc drugs:
Daunorubicin (daunomycin, rubidomycin)
Doxorubicin (Adriamycin)
Bleomycin (blenoxane)
Bleomycin (Blenoxane)
Bleomycin sulfate is a mixture of 13 different bleomycin peptides derived from a streptomyces spp.
Inhibits DNA synthesis, binds to DNA strands and causes DNA strand breaks
Chelates Fe2+ and bonds to DNA where it generates free radicals that degrades DNA
Can be inactivated by bleomycin hydrolase so has a spectrum of activity
Resistance conferred by increased hydrolase activity, decaresed uptake and increased efflux
Increased sensitivity in G2 phase
Causes fever, dermatologic reactions, pulmonary toxicity and fibrosis, minimal myelosuppression
Summary of resistance mechanisms
Decrease transport
Gene amplification
Increased DNA repair
Increase in deactivating enzymes
Modified enzymes
Multiple drug resistance —> a mechanism to pump out the drug
P-glycoprotein (p170)
ATP-dependent exporter
Broad spectrum of action
Overexpressed in resistant cells
These mechanisms of resistance are different from those in the targeted rug treatment as they try to reestablish survival pathways whereas here the cancer cells are trying to minimise interaction with the drug.
Resistance via efflux
tumour over express efflux pumps like ABC transporter and MDR1 to pump out chemotherapeutics such as taxanes, topoisomerase inhibitors and antimetabolites
even molecular targeted therapies like imatinib and nilotinib can be effluxed via MDR1 transporters
Resistance via DNA damage repair
many chemotherepeutics induce DNA damage and the cellular response to this is either repair DNA or induce cell death
Therefore, the ability of cells to repair damage has a major influence on the ability to induce cell death
whether cells die in response to DNA damage is largely dependent on the mitochondrial apoptosis pathway which is controlled by the BCL-2 family of proteins
BH3 priming describes how close cells are to the apoptitic threshold —> cells which are highly BH3 primed will have a lot of pro death signals already present so only a small amount of stress will cause it to die
Low BH3 primed cells will more likely resist chemotherapy and will keep the mitochondrial death pathways tightly blocked.
Combination therapy
Mechanism to reduce the dose, increase efficacy or delay the resistance
Greater range of side effects but less severe
Timeline for historical milestones for cancer treatments:

Epithelial-mesenchymal transition (EMT) driving resistance to chemotherapy
EMT is a biological programme used in embryonic development and wound healing which cancer cells hijack to become more invasive and develop resistance
during EMT cells lose tight cell-cell adhesion, gain mobility and flexibility
can be measured with molecular hallmarks such as the loss of E-cadherins, claudins and occludins, as well as the gain of fibronectin, vimentin and N-cadherin
EMt cells divide more slowly, have increased damage repair mechanisms and reduced dependence on the oncoprotein by activation of bypass pathways
They can also upregulate anti-apoptitic proteins as well as upregulate PD-1L and exclude cytotoxic T cells
After therapy, these cells can transition back to epithelial cells (MET) and can continue to proliferate —> this allows them to resist chemotherapy.