Chapter 11: Molecular Shape and Bonding Theories
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
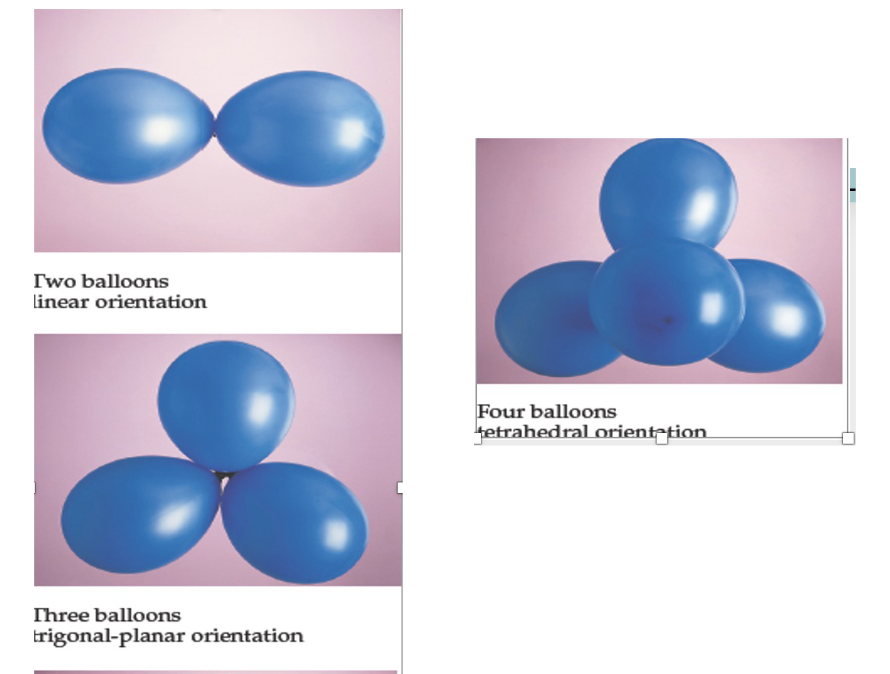
Valence Shell Electron Pair Repulsion Model
aka VSEPR: predicts the shape of a molecule based on the number of electron domains/electron group surrounding the. central atom
Each electron group is a charge cloud made up of either shared or lone-pair electrons
The most stable arrangement is one with the least repulsion btwn electron domains w/ the domains as far apart as possible while still connected to the central atom
Electron Domains or Electron Groups
Each of the following is considered one
electron group.
1. Each single bond
2. Each double bond
3. Each Triple bond
4. Each lone pair
The central atom in this molecule, A, has four
electron groups.
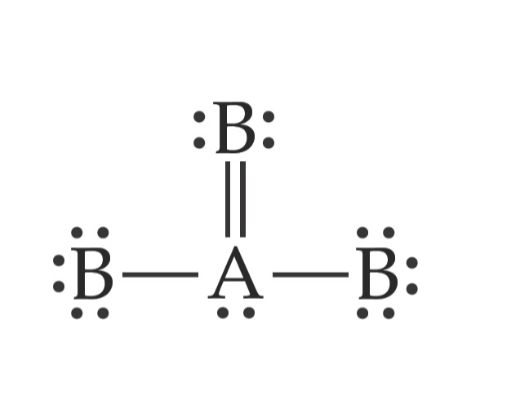
VSEPR Model
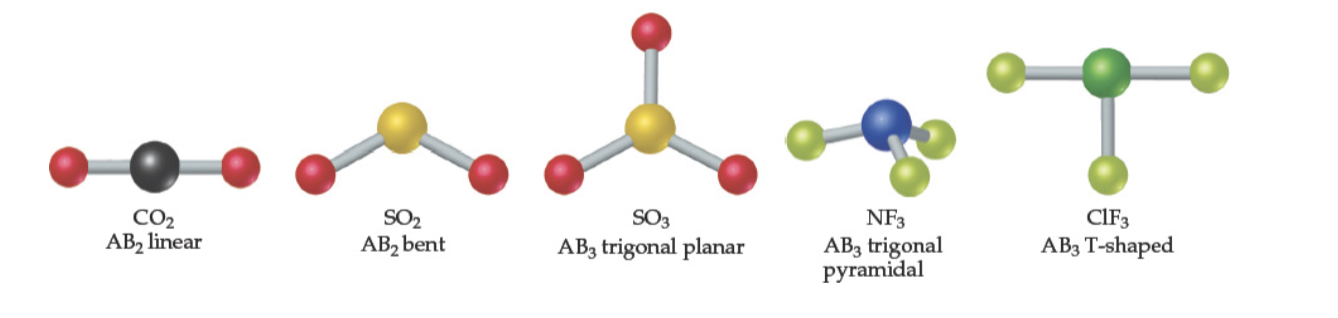
Molecular Shapes / Molecular Geometries
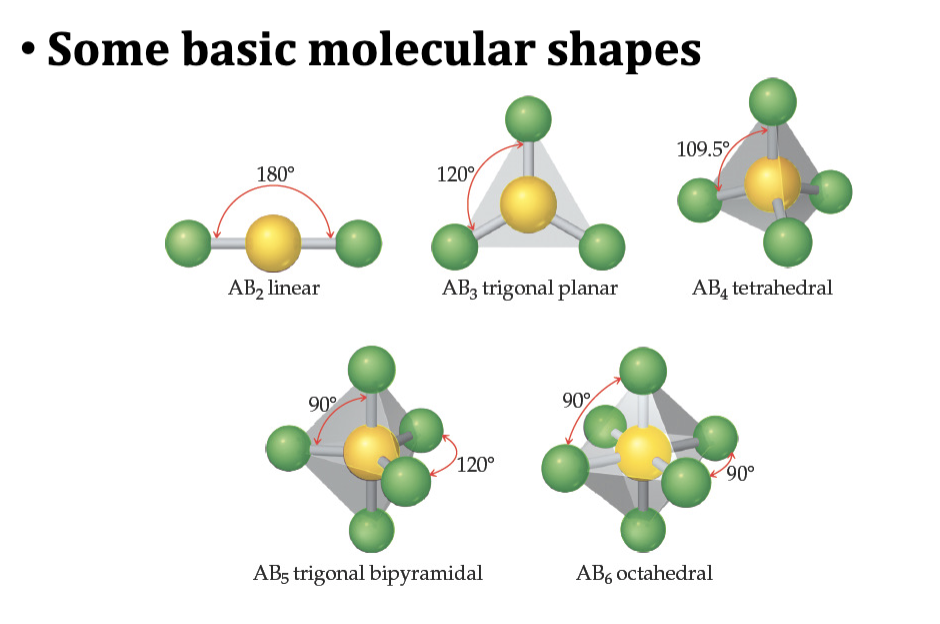
Memorize Tetrahedral Bond Angle
109.5
Lone Pairs v. Bonds
Lone pairs take up more space than bonds, this makes the bond angles smaller
Shapes of Larger Molecules
For larger molecules look at the geometry about each atom rather than the molecule as a whole
Any nonterminal atom can be considered a central atom
double bonds are considered as 1 pair
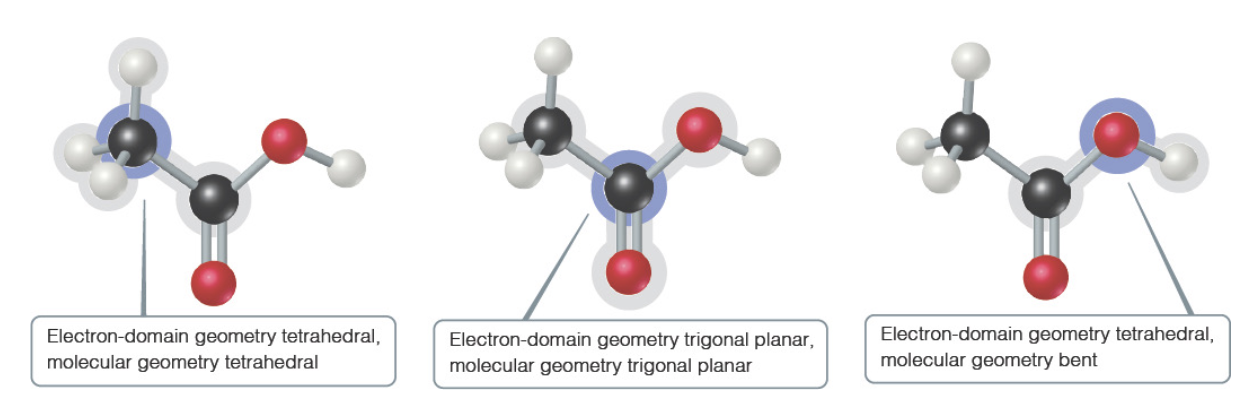
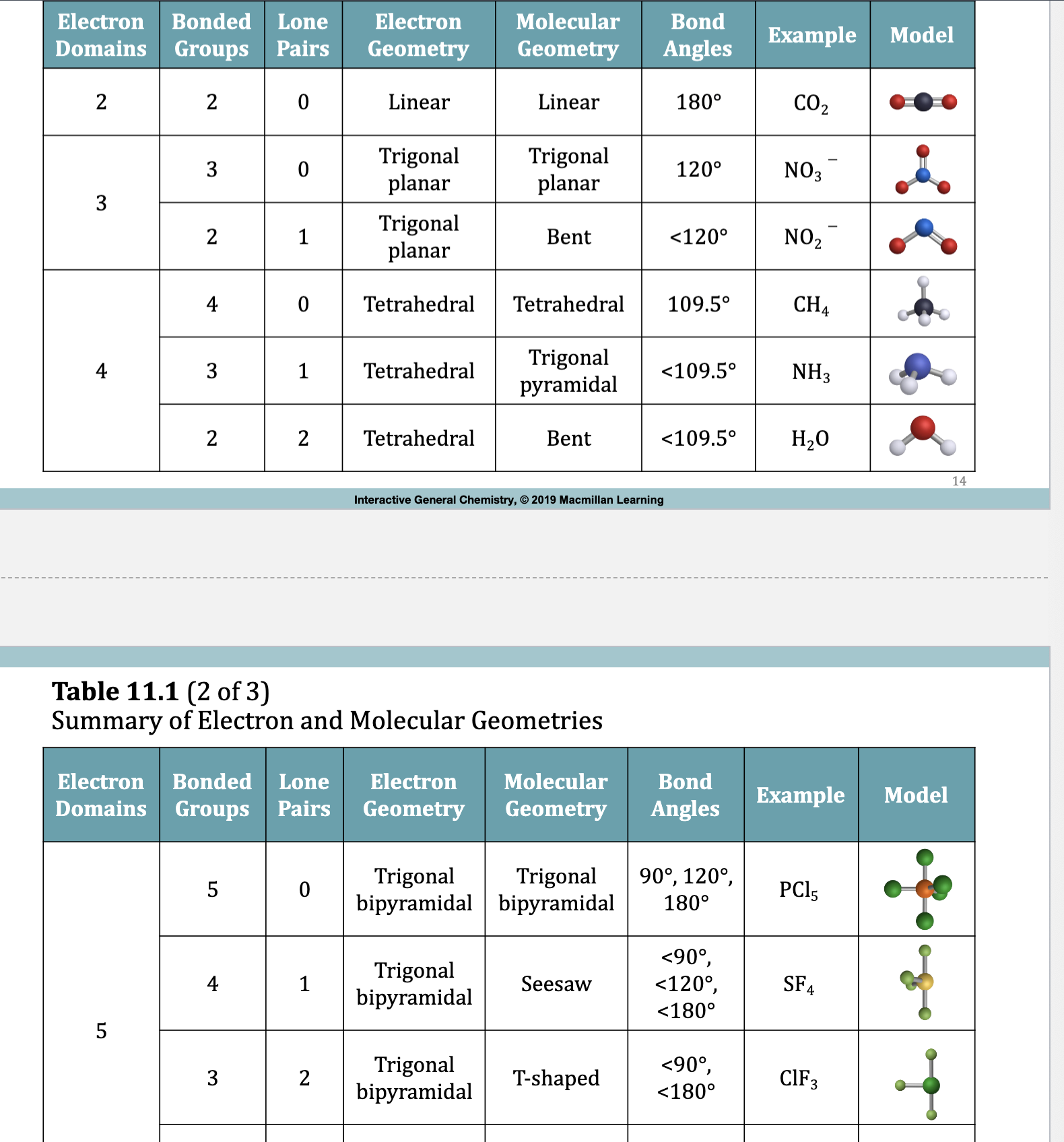
Table 11.1 Summary of Electron and Molecular Geometries
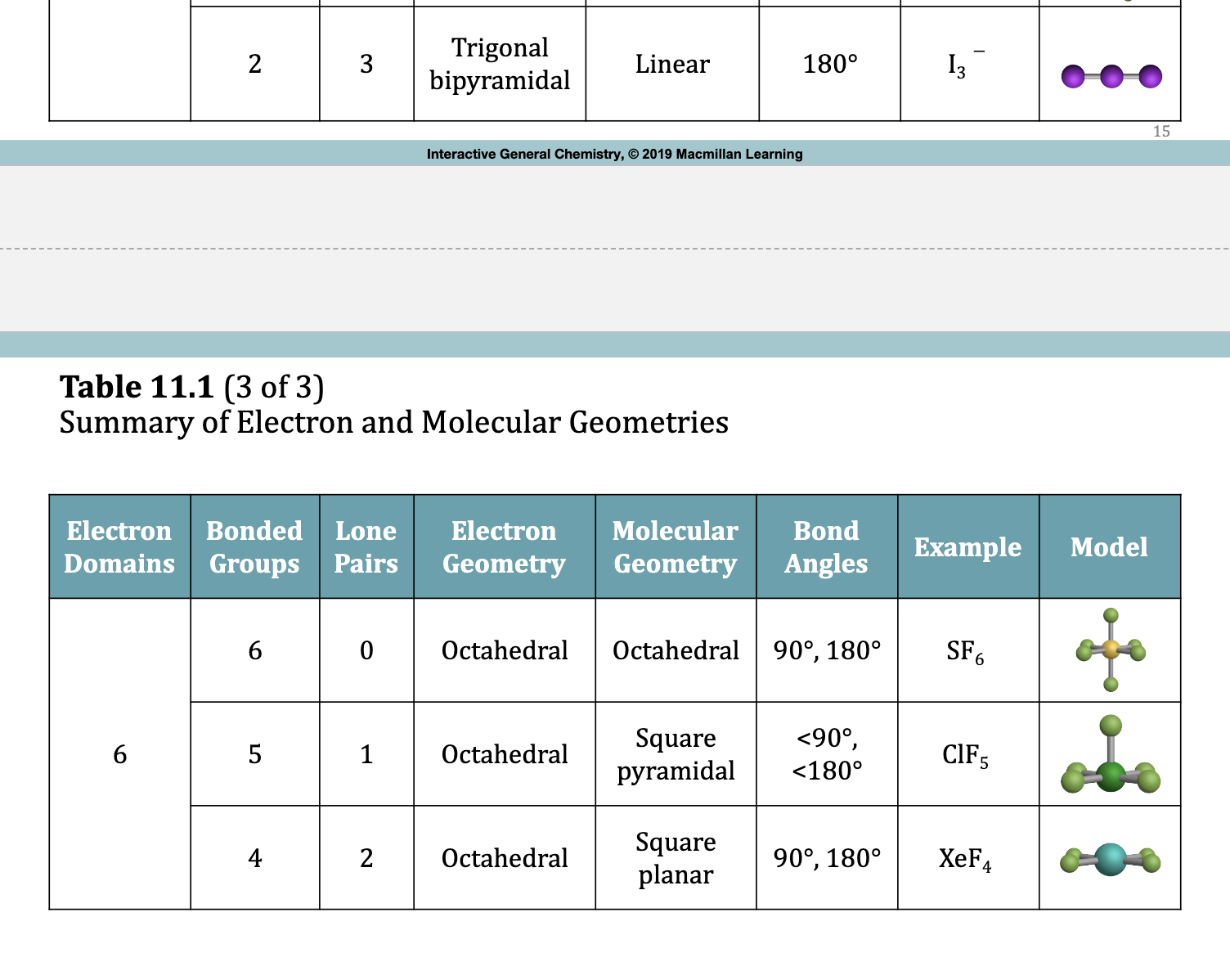
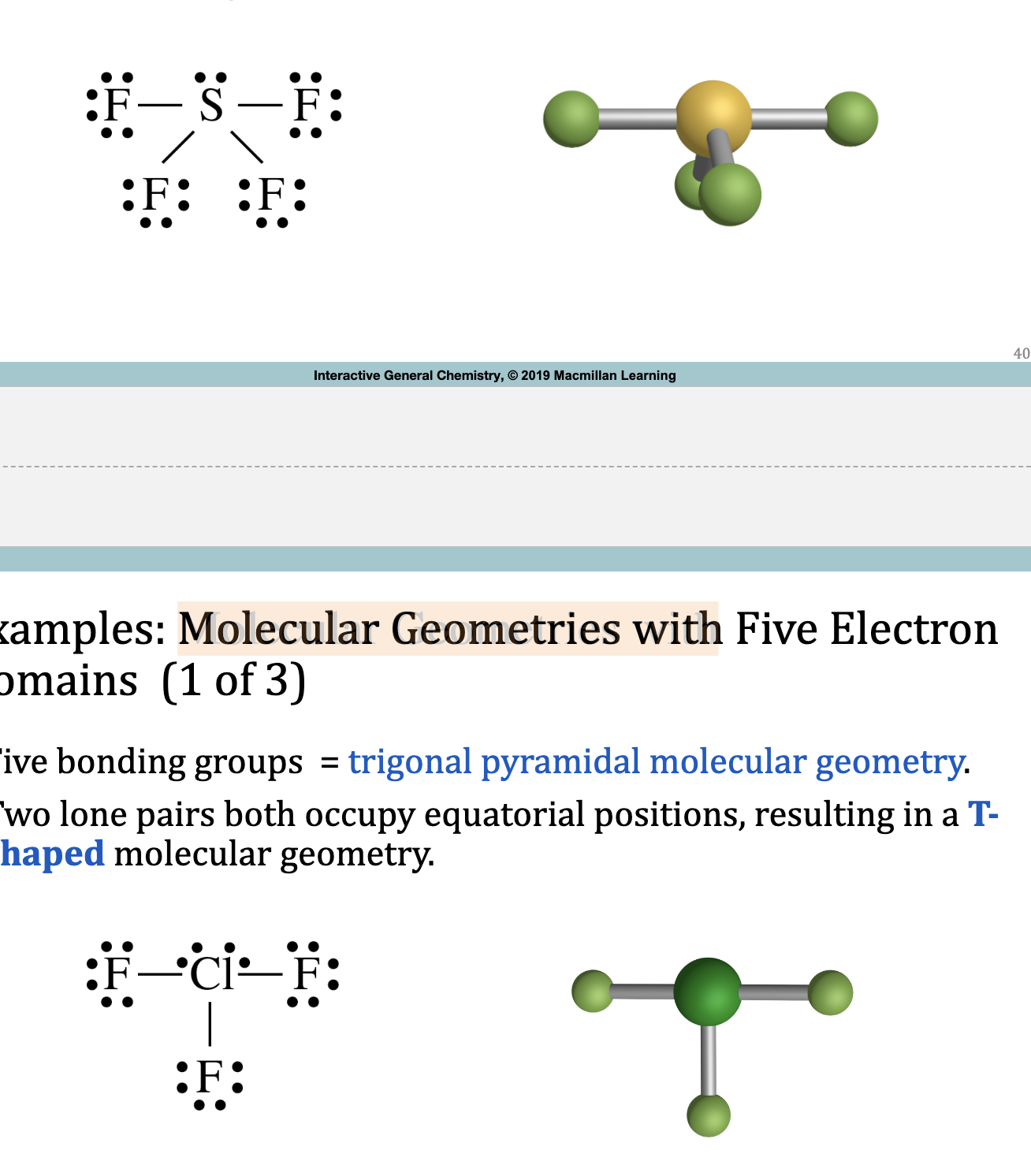
Trigonal Bipyramidal Electron Domain: Five Electron Groups Around the Central Atom
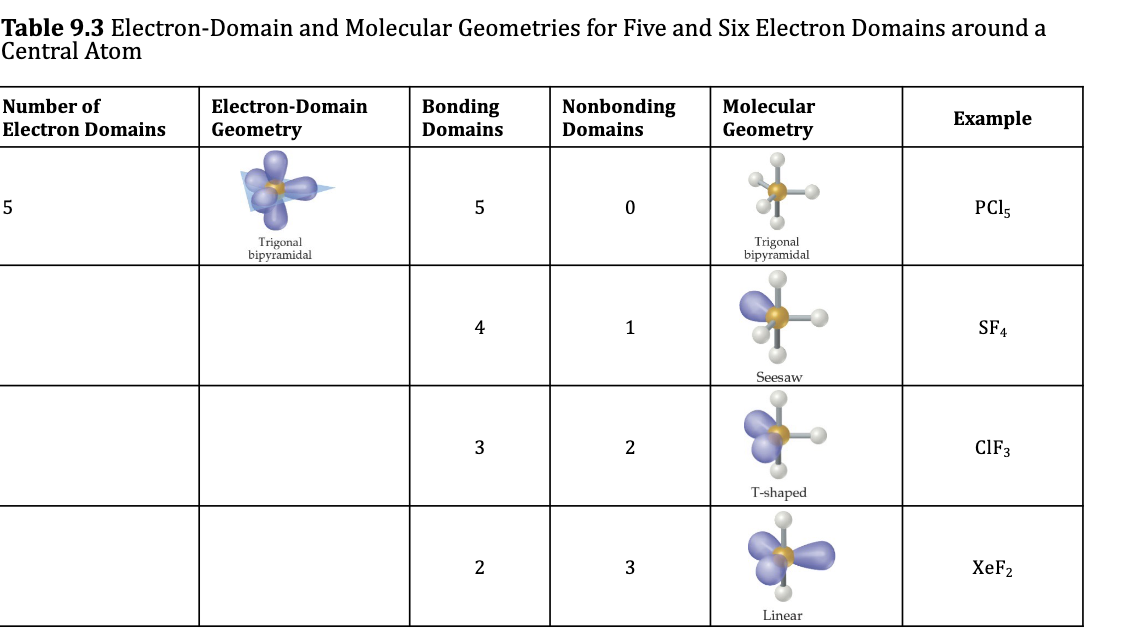
Examples: Molecular Geometries with 5 Electron Domainds
5 bonding groups = trigonal pyramidal molecular geometry
1 lone pair occupies an equatorial position = see-saw
2 lone pairs equatorial = T-shaped
3 lone pairs equatorial = linear
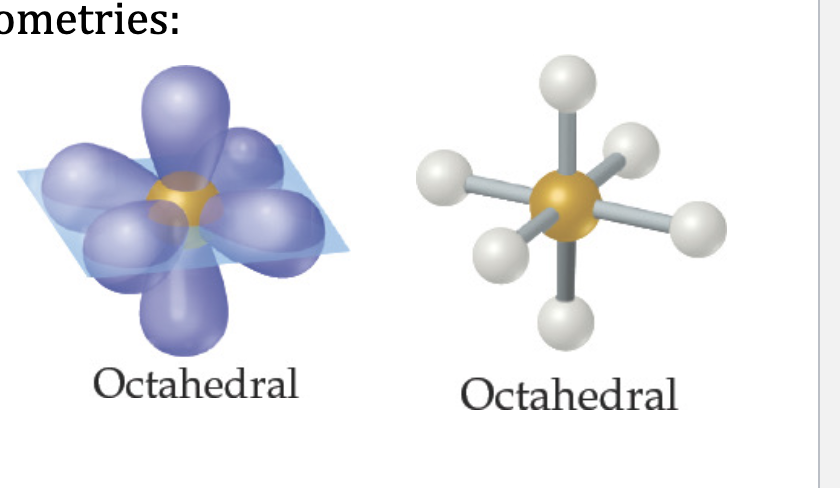
Octahedral Electron Domain: Six Electron Groups Around the Central Atom
• All positions are equivalent in the octahedral domain.
• All bond angles are 900.
• There are three molecular geometries:
1. Octahedral
2. Square pyramidal
3. Square planar
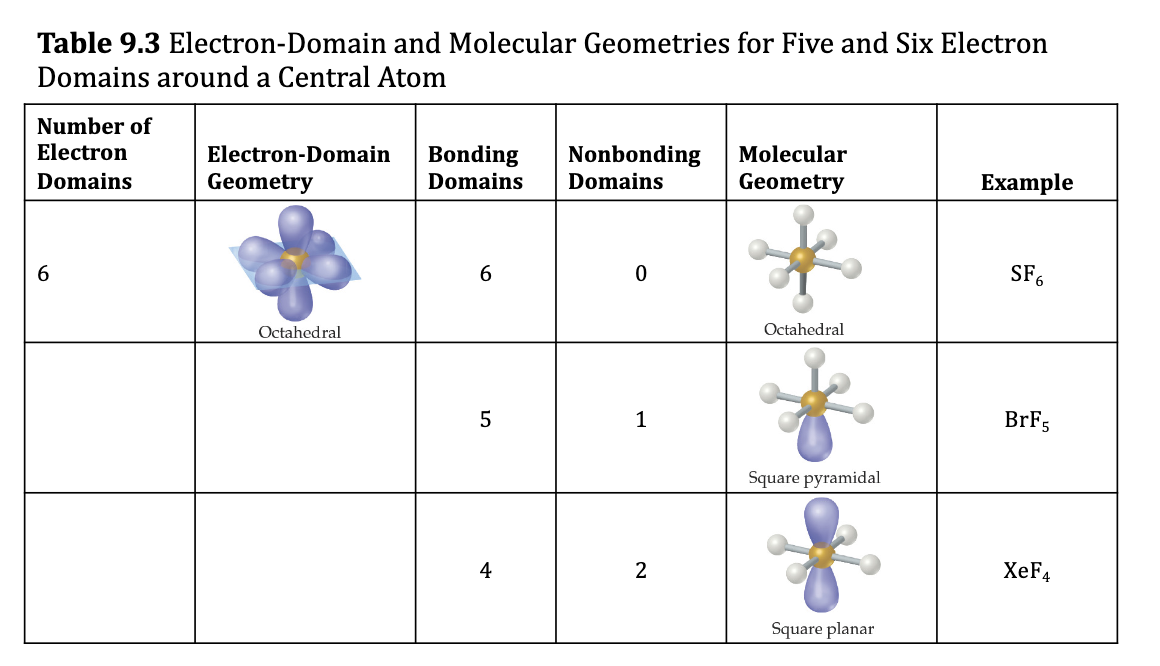
Octahedral Electron Domain: Six Electron Groups Around the Central Atom Table 9.3
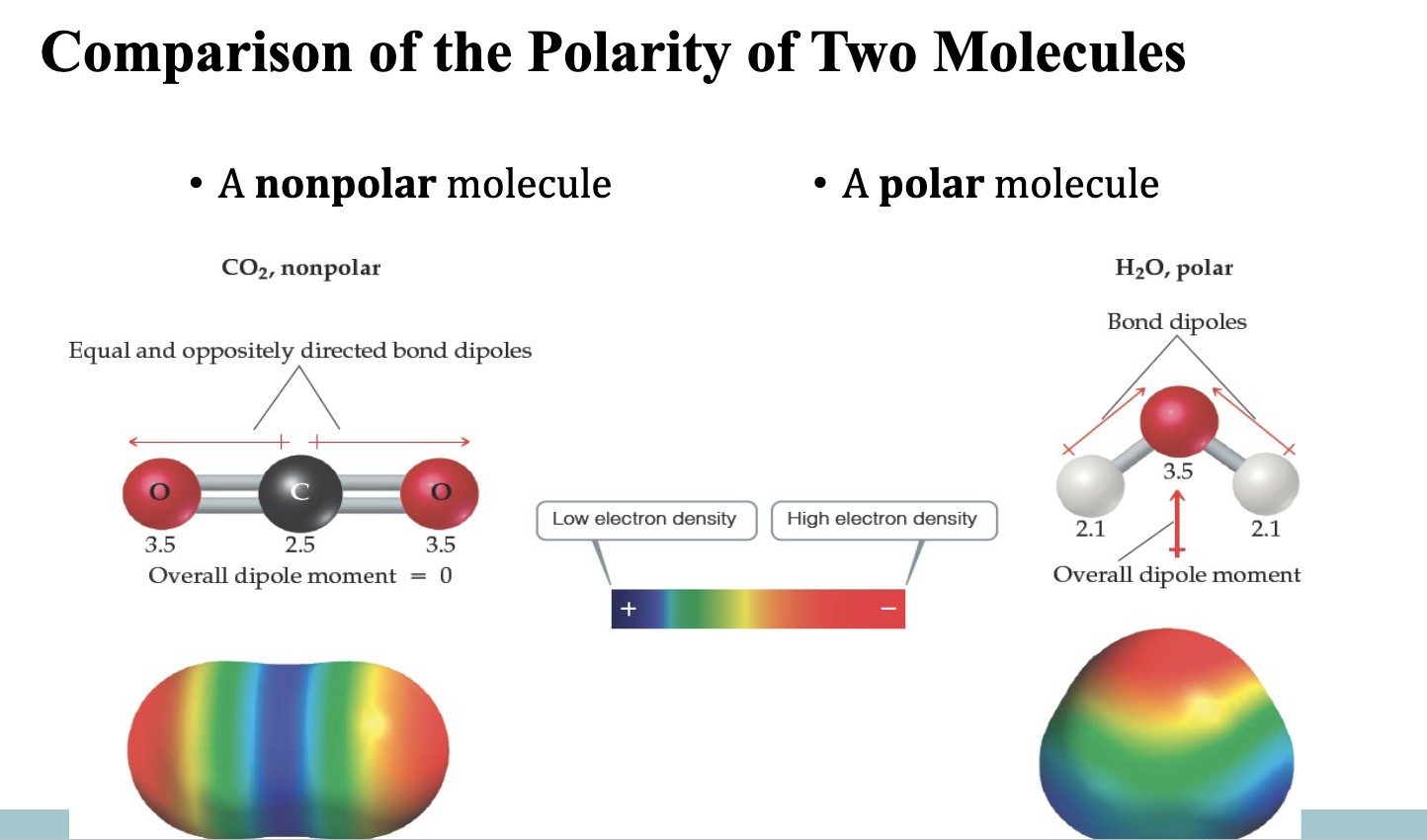
Polar and Nonpolar Molecules
1) Any molecule with onle 1 polar bond is a polar molecule
asymmetrical electron dist = polar
2) Any molecule with only ` nonpolar bond is a nonpolar molecule
symmetrical even if it has polar bonds
Comparison of the Polarity of 2 Molecules Figure
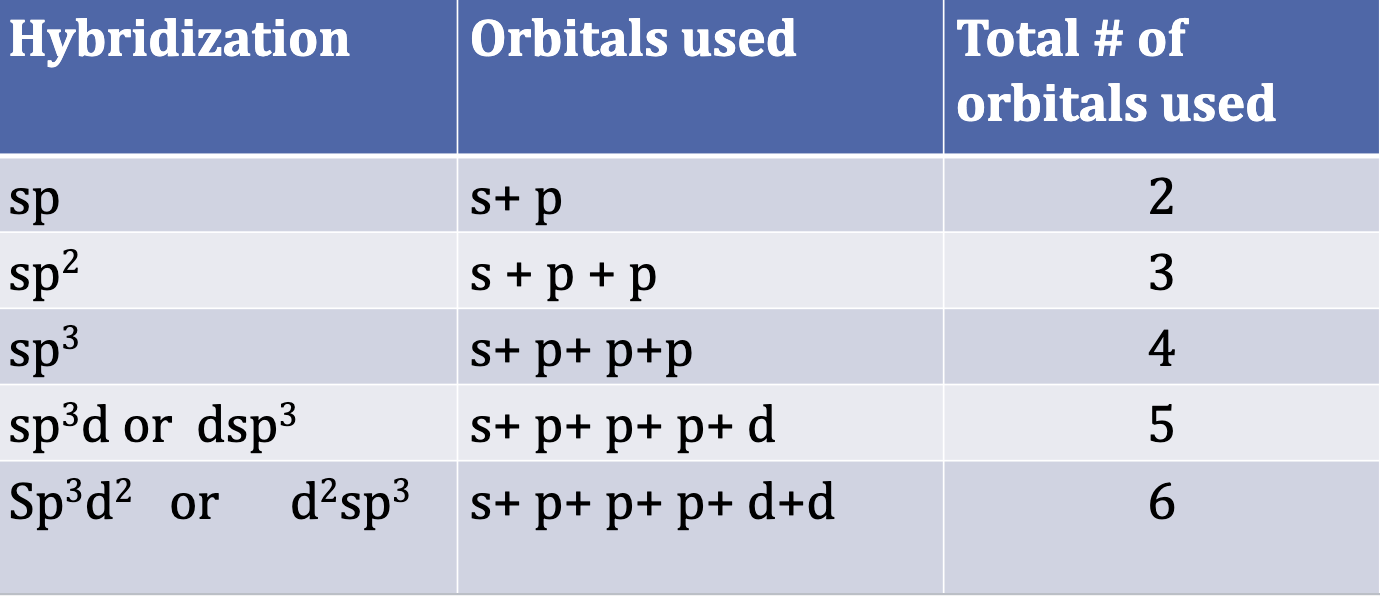
VB Theory: Hybridization of Orbitals and Bonding
Just count the number of electron groups in Lewis Structure for hybridization
Sigma Bonds
Sigma bonds are formed by head-to-head overlap
or end-to- end overlap of atomic orbitals.
• The overlap in these sigma bonds occurs around
and along the internuclear axis
every single bond = 1 sigma bond
double bond = 1 sigma + 1 pi
triple bond = 1 sigma + 2pi
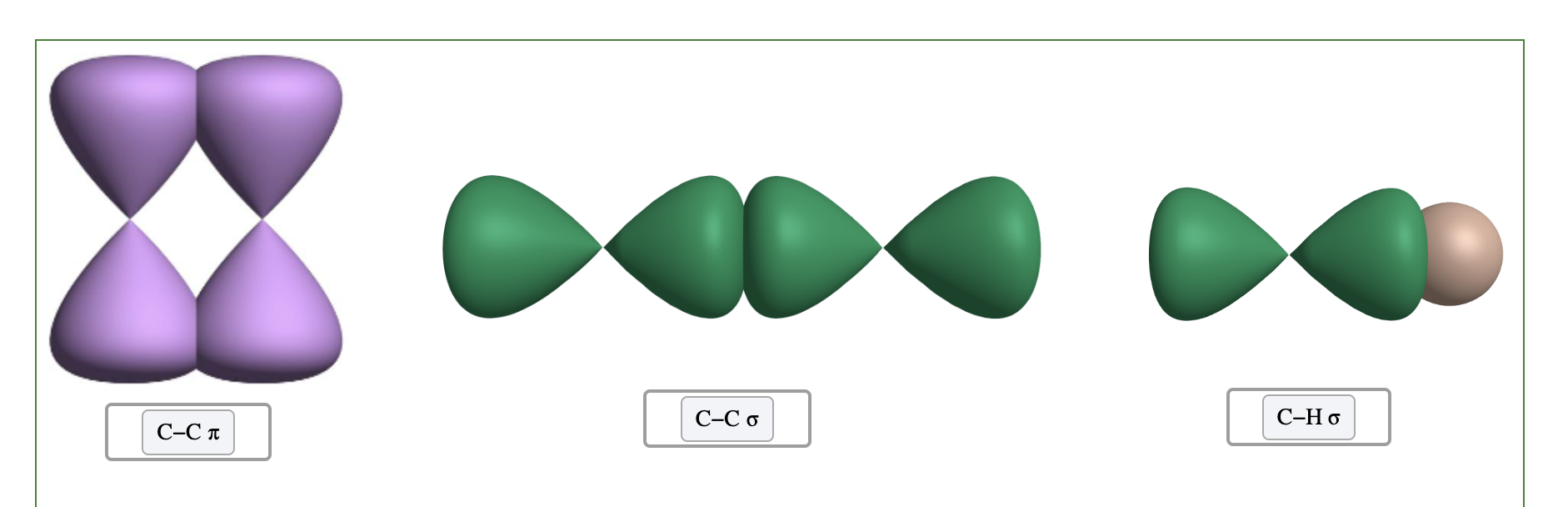
Pi Bonds
• Pi bonds are formed by:
– overlapping side-to-side.
– electron density above and below the internuclear axis.
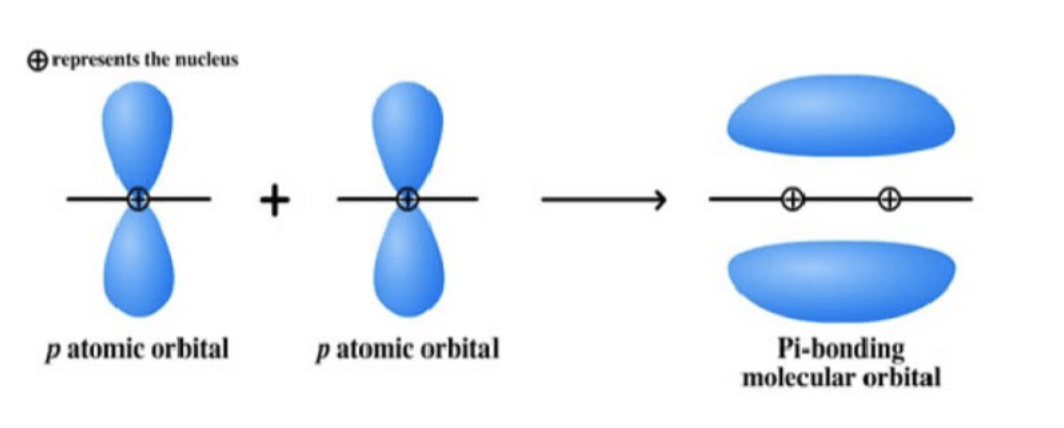
Sigma Bonds (head to head overlap) and Pi Bonds (side to side overlap)
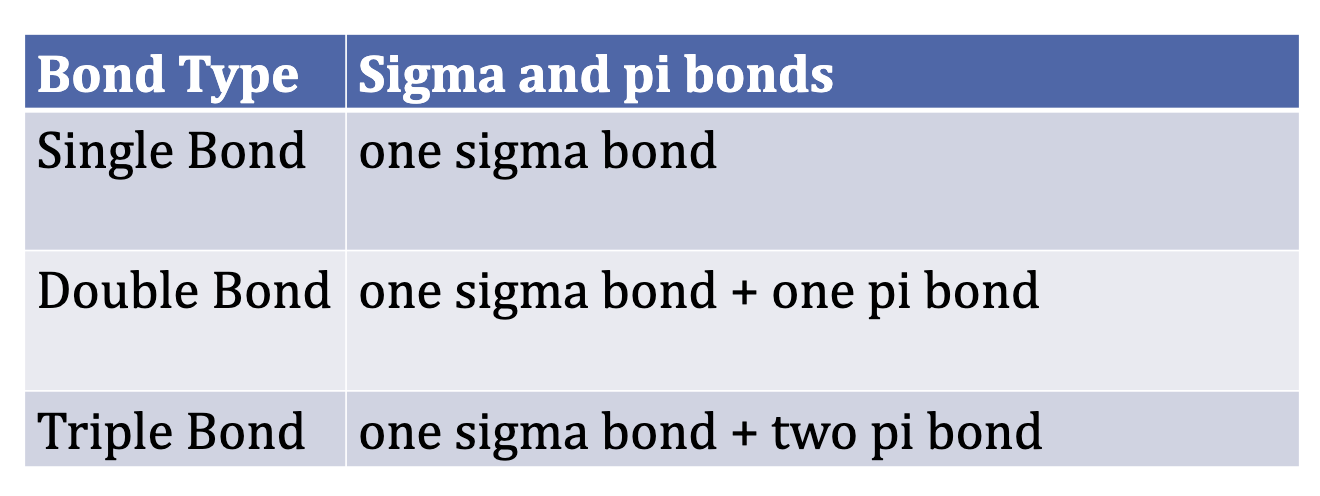
Valence Bond Theory
Atoms form bonds when their atomic orbitals overlap.
Each atom has electrons in orbitals, and when two atoms get close, one electron from each atom can pair up to form a bond.
The stronger the overlap, the stronger the bond.
Sometimes, orbitals mix or hybridize to form new shapes that explain the molecule’s geometry (like tetrahedral or linear shapes).
Bonding = orbital overlap + electron sharing.
How to find locations of negative pole
Look at the atoms in the compound that have the highest electronegativity
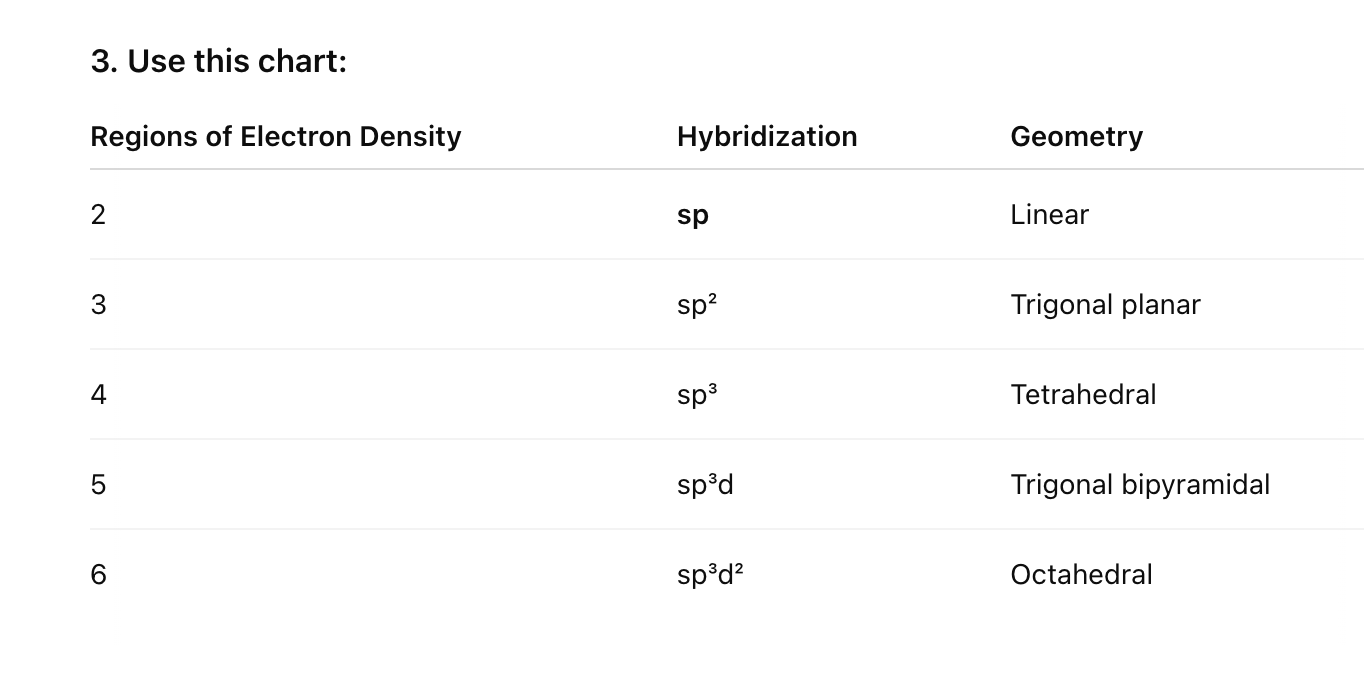
Hybridization
Draw the Lewis Struture
Count the regions of electron density (bonds + lone pairs) around central atom
Remember a triple bond = 1 region of electron density
How to Rotate Freely
Single bonds = rotate freely
Double or Triple Bonds = can’t rotate freely
How to determine if a molecular is polar from its lewis structure
1. Determine the molecular geometry.
Is the shape linear, bent, trigonal planar, tetrahedral etc
2. Look for polar bonds.
Check if there is a significant difference in electronegativity between the atoms in the bond.
Polar bonds occur when one atom is more electronegative than the other, creating a dipole moment (partial positive and negative ends).
Check if the polar bonds cancel each other out.
If the molecular geometry is symmetrical, the polar bonds may cancel out, making the molecule nonpolar.
Example: CO₂ is linear with two polar C=O bonds, but the symmetry makes the molecule nonpolar (dipoles cancel out).
If the geometry is asymmetrical, the polar bonds will not cancel out, making the molecule polar.
basically look at blue ws
Reactivity
Only single bonds = least reactive
Alkene w double bond = more reactive
alkyne w triple bond = most reactive
Polar Bonds
Look at atom electronegativity, bonds between two elements are polar if electronegativity is great (i.e. Xe and F are polar because F has high electronegativity than Xe) talked about in Ch 10
How To Determine a Large Net Dipole Moment
1. Check for Polar Bonds:
Polar bonds arise when there is a significant difference in electronegativity between two atoms in a bond.
2. Determine the Molecular Geometry:
The shape of the molecule (which you can deduce from its Lewis structure and VSEPR theory) plays a critical role in whether the bond dipoles cancel out or not.
If the molecule has a symmetrical geometry, the bond dipoles may cancel out, resulting in a nonpolar molecule despite the presence of polar bonds.
If the molecule has an asymmetrical geometry, the bond dipoles do not cancel out, and the molecule will have a net dipole moment.
3. Evaluate the Size of the Dipole Moment:
A larger difference in electronegativity between the bonded atoms usually leads to a larger dipole moment.
4. Assess the Overall Molecular Dipole Moment:
Once you determine whether the molecule has polar bonds and if the geometry leads to a net dipole, you can assess the net dipole moment of the molecule.
A molecule with asymmetrical geometry and strong polar bonds will have a large net dipole moment.
A molecule with symmetrical geometry, even if it has polar bonds, will have a small or zero net dipole moment because the dipoles cancel out.
Bond Length (review)
Triple bond = shortest
Double = medium
single = longest
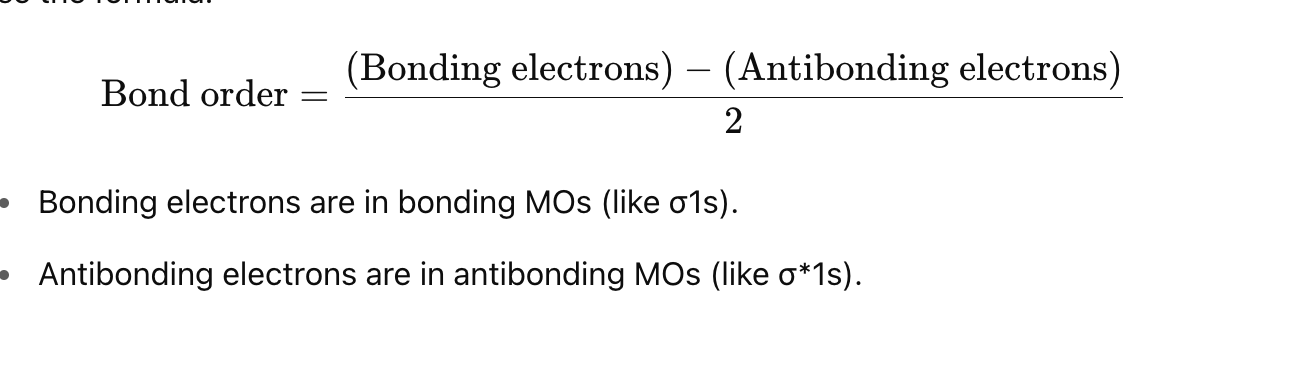
bond order in molecular orbital diagram
Steps to Find Bond Order:
Count the number of electrons in:
Bonding orbitals (like σ\sigmaσ, π\piπ)
Antibonding orbitals (marked with a star: σ∗\sigma^*σ∗, π∗\pi^*π∗)
Use the bond order formula:
Bonding vs. Antibonding Orbitals
Bonding orbitals: lower in energy, no star (σ1s,π2p\sigma_{1s}, \pi_{2p}σ1s,π2p)
Antibonding orbitals: higher in energy, have a star (σ1s∗,π2p∗\sigma^*_{1s}, \pi^*_{2p}σ1s∗,π2p∗)
Paramagnetic quick tip
If a molecule has an odd number of electrons, it is automatically paramagnetic because there must be an unpaired electron!
Molecular Orbital Theory
based on quantum mechanical principles
atomic orbitals of diff atoms combine to form molecular orbitals that are delocalized (spread) over the resulting molecule
2 atomic orbitals combine to form 2 molecular orbitals (1 bonding and 1 antibonding)
Molecular Orbital Theory: Additive Combination
of the wave functions of atomic orbitals results in a bonding molecular orbital
Molecular Orbital Theory: Subtractive Combination
of the wave functions of atomic orbitals results in an antibonding molecular orbital.
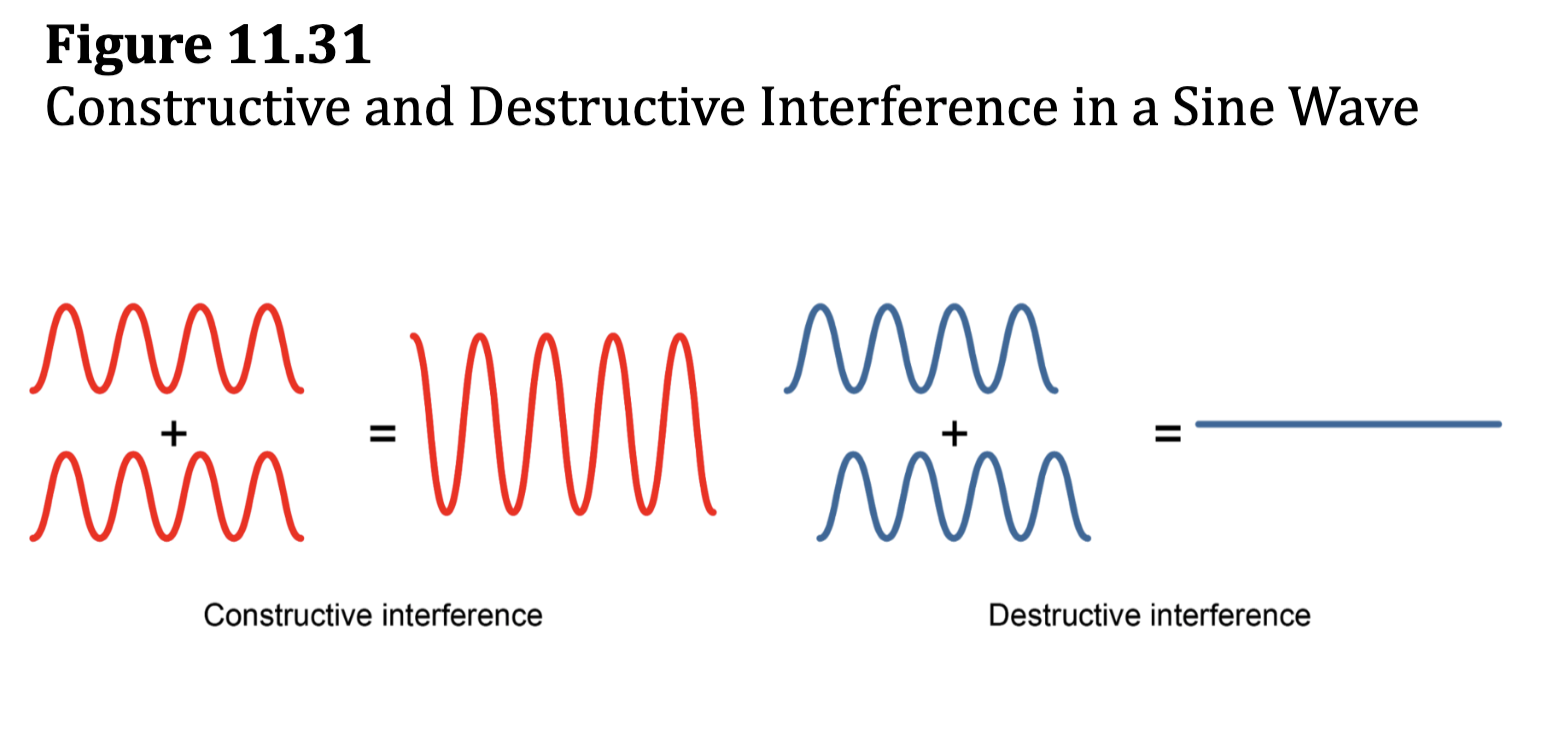
Fig. 11.31: Constructive and Destructive Interference in a Sine Wave
subtractive combo = destructive interference = antibonding
additive combo = constructive interference = bonding
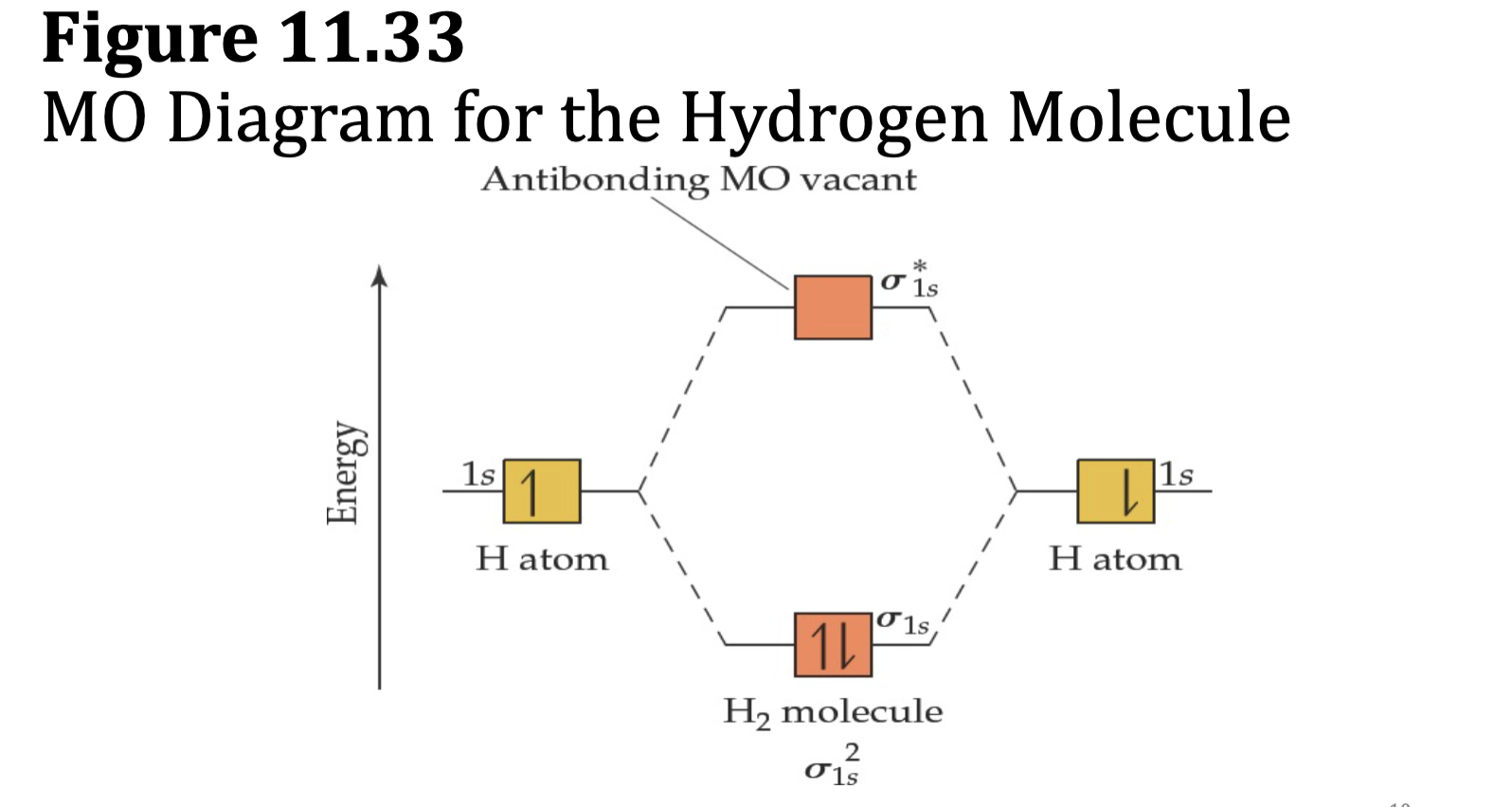
Molecular Orbitals and the H2 molecule
• When two H atoms come together, the 1s orbitals from each undergo additive combination to form a σ1s bonding molecular orbital, which:
• has the highest probability of electron density along the
internuclear axis,
• has lower energy than the 1s atomic orbitals, and
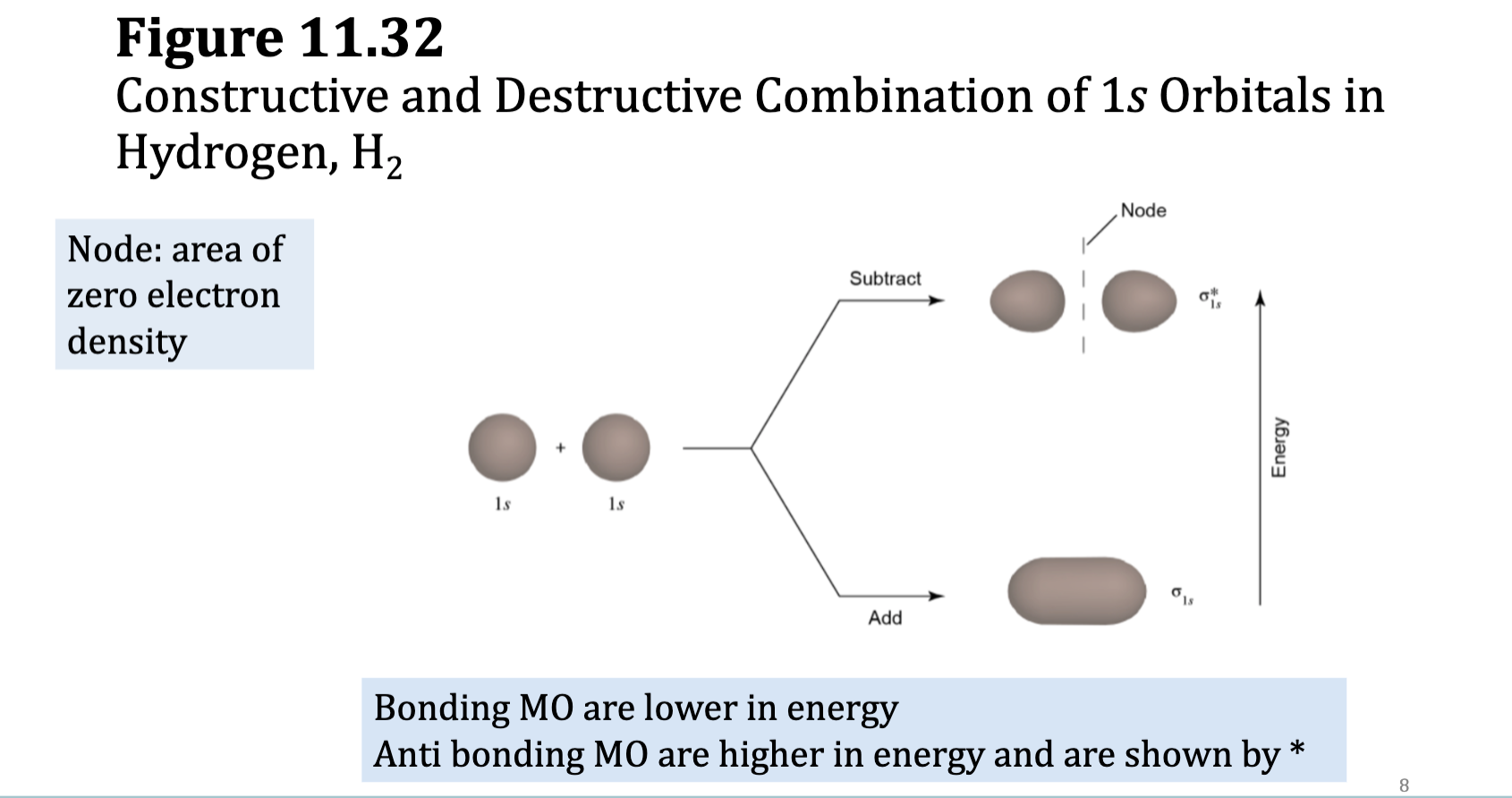
Figure 11.33: MO Diagram for the Hydrogen Molecule
Bond Order Formula
½ (# of bonding electrons - # antibonding electrons)
A BO of 1 = single bond in a Lewis structure
A BO of 0 = bond has no stability
Paramagnetic vs. Diamagnetic
Diamagnetic: all electrons in MO Diagram are paired
Paramagnetic: some electrons in MO Diagram are unpaired
Molecular Orbital Diagrams
• A molecular orbital, MO, diagram shows how the molecular orbitals are populated with electrons.
• The lowest energy levels are filled first, following the aufbau principle.
• These diagrams can then be used to write molecular electron configurations.
Combining the p Orbitals
• Only one set can combine to overlap along the internuclear axis to form σ bonding and antibonding MOs.
• The other two p orbitals combine sideways to form π bonding and antibonding orbitals.