IB HL Biology: Water
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Water as the medium for life
Life began in water as it was the first cells in the ocean.
- Small droplets of water enclosed in a membrane forming a vesicle
- substances then dissloved in this water allowing chemical reactions to occur between solutes
- after billions of years of evolution, most molecules used for processes of life are still dissolved in water where they can interact and move around
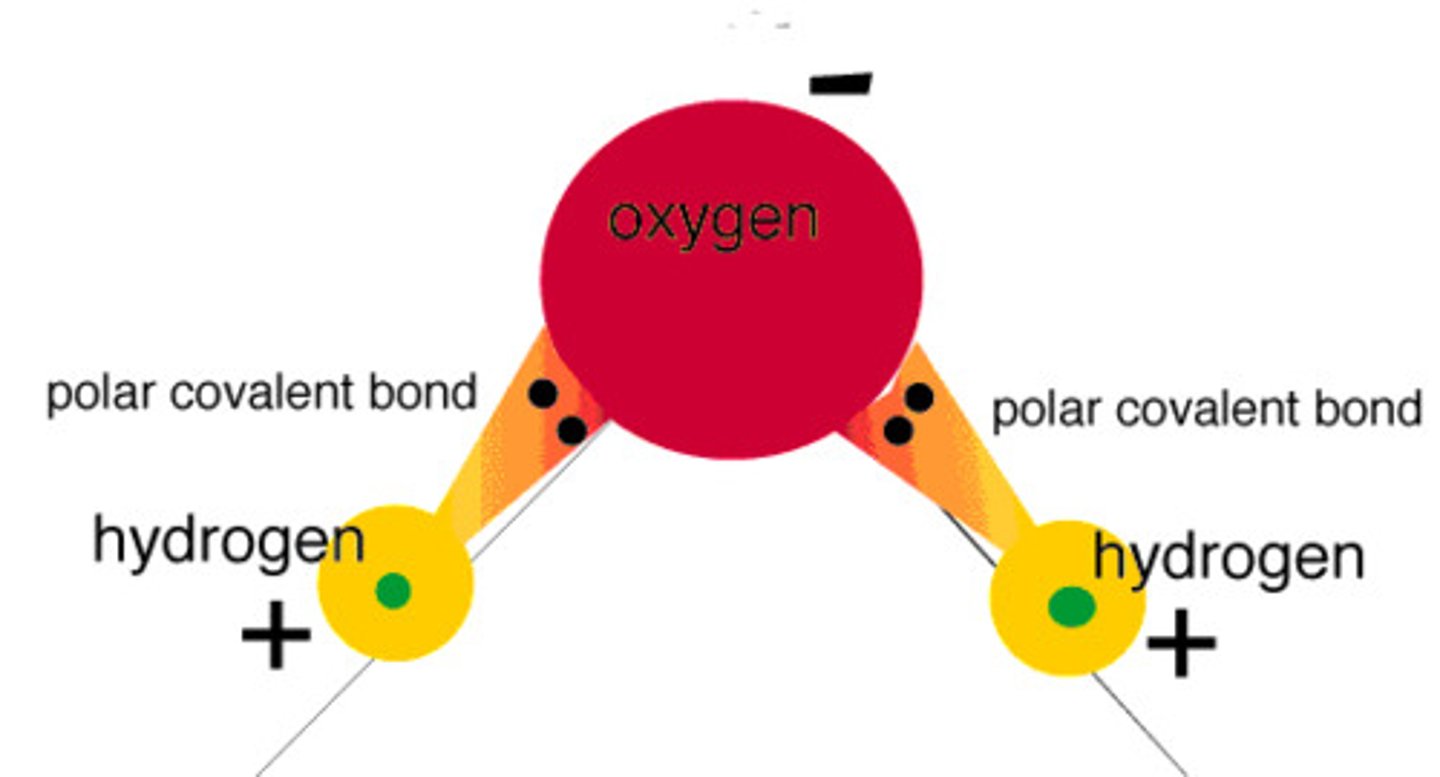
Hydrogen bonds as a consequence of the polar covalent bonds within water molecules
- Covalent bonds between oxygen and hydrogen
- sharing of electrons in these bonds (covalent) are unequal so they are polar (partial positive charge and negative charge in the molecule) hydrogen atoms have a partial positive charge and oxygen atom has a partial negative charge
- because the nucleus of an oxygen atom is more attractive to electrons than the nucleus of a hydrogen atom due to an oxygen atom having more nuclear charge
seperation of charge caused by unequal sharing of electrons in covalent bond is called a dipole
Water molecule structure:
- Bent
- 2 hydrogen atoms on the same side of the molecule forming the opposite pole
- oxygen atom on the other side form th opposite pole
- positively charged particles (positive ions, hydrogen ions) and negtaively charged particles ( negative ions, oxide ions) attracted to each to eachother forming ionic bonds between hyrdrogen and oxide ions
Hydrogen bond
Intermolecular force that ahppens when a slightly positive hydrogen atom from one pole is attracted to a slight negative oxide ion of another polar molecule
Cohesion
- Water molecules sticking together due to mutual attraction caused by hydorgen bonds between them (IMF)., Energy is required to break these bonds
- Hydrogen bonding between the water molecules allows the liquid to resist low levels of external force (surface tension)
- High surface tension makes it suffiecnely dense for smaller organisms to move along its surface
cohesion of water under tension in xylem
Use of water surfaces as habitats
- all liquids have surface tension which is when the surface of water acts as an elastic membrane that shrinks to the minimum possible area because water molecules are more attracted to each other by hydrogen bonding than to air particles
- surface tensions allows some objects to float on the surface even if they are dense and may sink because cohesion between water molecules due to hydrogen bonds is greater than the attraction beetween the water molecules and the object
- for an object to break throguh the surface tension of water, many hydrogen bonds would need to broken simulteanously using enough energy
- Living organisms make use of this proeprty of water by using it as a habitat:
- Water striders walk on the water with their 6 legs
- Mosquito larvae live just below the surface, using their siphon to hang on
Adhesion
- Attraction between water molecules and the surface of a solid composed of polar molecules, causing water to stick to the surface of this solid
Capillary action:
When adhesion causes movement when water is drawn through narrow glass tubes because when water replaces air along the glass tube, hydrogen bonds are formed between glass and water which are stronger than hydrogen bonds between water, so energy is released and water is pulled up by stronger attraction of adhesion between water and polar glass tube surface.
Adhesion in paper
Porous solids such as paper have large amounts of surface area attractive to water (polar, due to containing cellulose, polymer of simple sugar glucose) so they can exert strong suction forces through adhesion.
We can see this when water is drawn through the narrow spaces between cellulose molecules in paper towels (pores) so paper becomes wet due to absorption of water.
Adhesion in soil
Water is attracted to many chemical substances (polar) in soil.
If soil is porous, water is drawn by capillary action through dry soil, wetting it. Water is pulled by suction forces where there is strong adhesion between a polar substance and water which is stronger than hydrogen bonds between water only causing water to rise up from the underground, even though gravity tends to pull it down.
Adhesion in plants
Water adheres to cellulose molecules in cell walls, so any cell wall that starts to dry out is automatically rewetted as long as there is a source of water available due to the attraction from adhesion between cellulose in the cell wall and water.
If water evaporates and is lost to the atmosphere from the cell walls in leaves, adhesive forces cause water to be drawn out of the nearest xylem vessel (water goes up). This keeps the walls moist so carbon dioxide gas can dissolve into the water of the plants to be absorbed for photosynthesis. It also generates the low pressures that draws water up in xylem vessels.
If a xylem vessel becomes air filled, adhesion between water and the wall of the vessel can help the vessel to refill with water.
For example, xylem vessels in deciduous trees (leaves fall off in autumn, brown orange leaves) are air filled through the winter. In spring, capillary action due to adhesion with water causes sap to rise, refilling the vessels with liquid, replacing the air.
Paraphyllia: Narrow hair-like structures on the stems of some mosses, in which the cellulose cell walls of the paraphyllia attract water from fog or dew and store it to keep the moss hydrated.
Water as a solvent
Hydrophilic
Polar substances or substances with a positive or negative charge which dissolve in water are hydrophilic meaning they are attracted to water and other hydrophilic substances (eg. glucose, sodium ion, chloride ion)
Substances that water adheres to but does not dissolve into (eg. cellulose) are also hydrophilic.
Hydrophobic
Non-polar substances or substances without a positive or negative charge are hydrophobic meaning they are not attracted to water but more attracted to other hydrophobic substances (eg. all lipids, including all fats and oils)
These are insoluble in water although they may dissolve in other solvents (eg. propanone (acetone)).
Metabolism:
Metabolism refers to the many different chemical reactions catalysed by dissolved enzymes in the cytoplasm to produce energy for the cell.
The cytoplasm is a complex mixture of dissolved substances and is an aqueous solution (water is solvent).
Because the cytoplasm is an aqueous solution and substances can dissolve in it (separate and disperse) solutes in this aqueous solution can move around and interact, allowing the components of chemical reactions for metabolism to move and bind to active sites of enzymes.
Thus water is the medium of metabolism
buoyancy
Buoyancy refers to the ability of an object to float in water
To overcome the problem of buoyancy, the black-throated loon has solid bones, unlike the hollow bones that most bird species have to assist them with flight
This increases the weight of the bird and compresses air out of the lungs and feathers during a dive
For the ringed seal, the layer of blubber under its skin will improve the buoyancy of the animal, along with providing a layer of insulation against the cold temperatures of its habitat
Specific Heat Capacity
The heat required to raise the temperature of 1 g of a material by 1°C (or kelvin, K).
The specific heat capacity of water is 4.18Jg(^-1)K(^-1). For air, the value is only 1.01Jg(^-1)K(^-1).
Water has a relatively high specific heat capacity because hydrogen bonds restrict molecular motion. For the temperature of water to increase, hydrogen bonds must be broken and energy is needed to do this. This is why a relatively large amount of heat is needed to raise the temperature of water. To cool down, water must lose an equally large amount of energy. As a result, the temperature of water remains relatively stable compared with air temperatures and aquatic habitats are more thermally stable than terrestrial habitats. The high specific heat capacity of water also helps birds and mammals (which are mostly composed of water) to maintain constant body temperatures.
viscosity
Viscosity refers to the resistance of a fluid to flow
The viscosity of water is much higher than air, which enables the black-throated loon to fly through the air without much friction
The body shapes of both the loon and seal makes it easy for them to move through water
Both organisms are adapted in their own way for movement through water:The seal has flippers to propel itself The loon uses its webbed feet to push against the water and the lateral location of its feet reduces drag as it moves through water
thermal conductivity
Thermal conductivity refers to the ability of a substance to conduct heat
The thermal conductivity of water is almost 30 times higher than that of air, which makes air a very good insulator for organisms living in colder climates
The black-throated loon (Gavia arctica) is a species of diving bird which spends much time underwater catching its prey
Their feathers trap an insulating layer of air, which assists them with regulating their body temperature
The seal on the other hand, relies on a layer of fat called blubber to insulate it from the outside air
Ice in its environment will also form an insulating layer above the water, since the thermal conductivity of ice is much lower than liquid water
This increases the sea temperature below the ice as thermal energy is trapped
cohesion of water under tension in xylem
- Cohesion allows transport of water under tension in plants
- Water is sucked upwards from the roots to the leaves through the tubular vessels in the xylem tissues due to the diffference in pulling forces from the leaves and from forces in the root
- Vessels are continuous columns containing water where each vessel containing water is under tension (pulling forces) whcih is developed as water is lost from evaportation to the surroundings so water moves from an area of high concentration to low concentration up the vessel
- due to attraction between water molecules and cells walls in leaf cells, water moves upwards as the pulling forces in the leaves are greater than pulling forces in roots