experiment 5: Michaelis Menten kinetics and enzyme inhibitions
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
what does the rate at which enzymes convert substrate to product depend on
the concentration of the substrate surrounding the enzyme.
-with more substrate, the rate of the conversion of substrate to product also increases.
more substrate present
the more substrate that is present, the less time it takes for a new substrate molecule to diffuse into the active site of the enzyme following the departure of the product molecules(s) from the previous reaction cycle
effect on V0 - low substrate concentrations
-catalytic rate is roughly proportional to substrate concentration
when substrate concentration is increased...
this increase in rate becomes less pronounced.
-the time required for a new substrate molecule to diffuse into the active site is decreasing, but the time required for substrate recognition and conversion to product is unchanging.
-at some very high substrate concentration, the time required for a new substrate molecule to enter the active site is approximately zero
saturated
-at some very high substrate concentration, the time required for a new substrate molecule to enter the active site is approximately zero, meaning that the active site of the enzyme is continuously occupied by either a substrate or product molecule, or some kind of intermediate that is formed during the catalytic mechanism.
saturation kinetics curve
-for many enzymes, this saturation kinetics follows a rectangular hyperbola curve, with one asymptote at Vmax which is the catalytic rate when the enzyme is saturated with substrate.
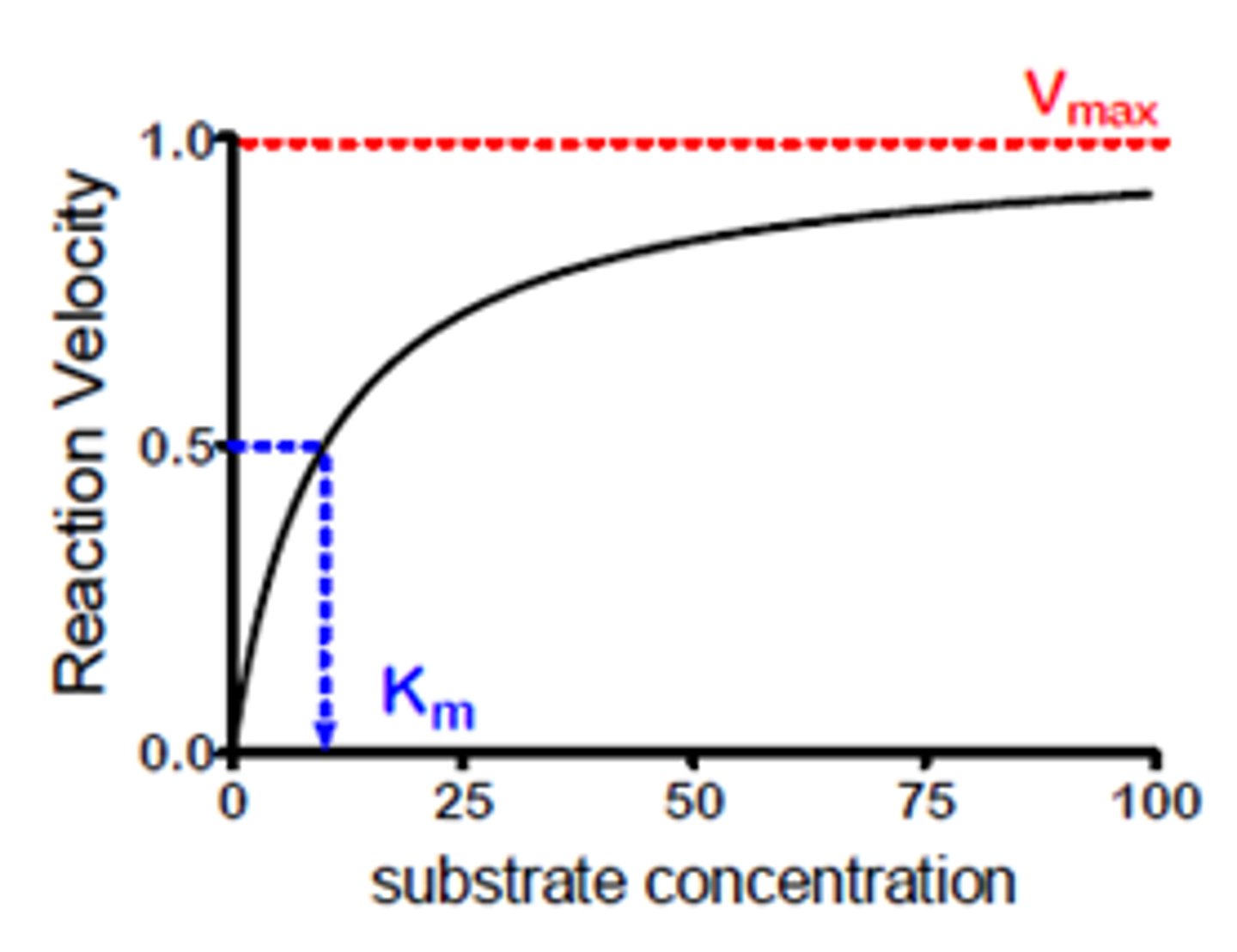
saturation kinetics curve in early 20th century
this kinetic information was used to formulate the hypothesis that enzyme and substrate formed an enzyme-substrate (ES) complex that was a prerequisite intermediate step for successful catalysis.
mathematical model
the saturation behaviour of these enzymes was generalized into a mathematical model
Michaelis Menton kinetics
-Leonor Michaelis and Maud Menten proposed that enzymes display this relationship between rate and substrate concentration are said to follow Michaelis Menton kinetics
Michaelis-Menten plot
a graph that depicts this relationship between V0 and [S] in this manner is known as a MM plot.
Michaelis Menten rate equation

Michaelis-Menten constant
km
-has units of concentration
-is a composite of several rate constants over the catalytic cycle.
what is km
it is the substrate concentration that generates an initial rate equal to 1/2 of the maximum rate
-km is characteristic for a particular type of enzyme under specified reaction conditions and is independent of enzyme concentration.
-under certain circumstances, the Km can represent the affinity of an enzyme for its substrate.
levelling off portion of a reaction progress curve
where equilibrium has been reached
levelling off portion of MM graph
due to saturation of the enzyme with substrate.
Km and Vmax
-they provide basic information about reactions.
-important for understanding enzymes.
whether a particular enzyme plays a role
-Km and Vmax are useful parameters to establish when studying enzymes.
-as part of the elucidation of a metabolic pathway it is often necessary to establish whether a particular enzyme plays a role. if Km (measured in vitro) is high compared with [S] in vivo, then it is unlikely to be an important reaction because very little substrate would be converted to product under prevailing physiological conditions
isozymes
-Km and Vmax are useful parameters to establish when studying enzymes.
-these parameters may also be different for different forms (isozymes) of the same type of enzyme. when these isozymes are distributed amongst different tissues, it enables the specific metabolic needs of the tissue to be met without requiring a completely different enzyme.
before modern computer based graphing, determining Km and V0 was impractical
-the substrate may not be soluble at high concentrations which would make the determination of Vmax impossible
-attempting to establish Vmax would be a waste of time and materials because you have to do a large number of assays to be confident that you have come close to the maximum initial rate.
solving this problem
-this practical problem is traditionally circumvented using linear transformations of the Michaelis-Menten rate equation with easily obtained experimental data.
Lineweaver-Burk equation
-this equation is for a straight line (y=mx +b) where 1/V0 are the y values, 1/[S] are the x values, Km/Vmax is the slope of the line and 1/Vmax is the y intercept.
![<p>-this equation is for a straight line (y=mx +b) where 1/V0 are the y values, 1/[S] are the x values, Km/Vmax is the slope of the line and 1/Vmax is the y intercept.</p>](https://knowt-user-attachments.s3.amazonaws.com/649a00c9-7549-4c19-b057-5d4131e307d1.jpg)
typical lineweaver-burk plot
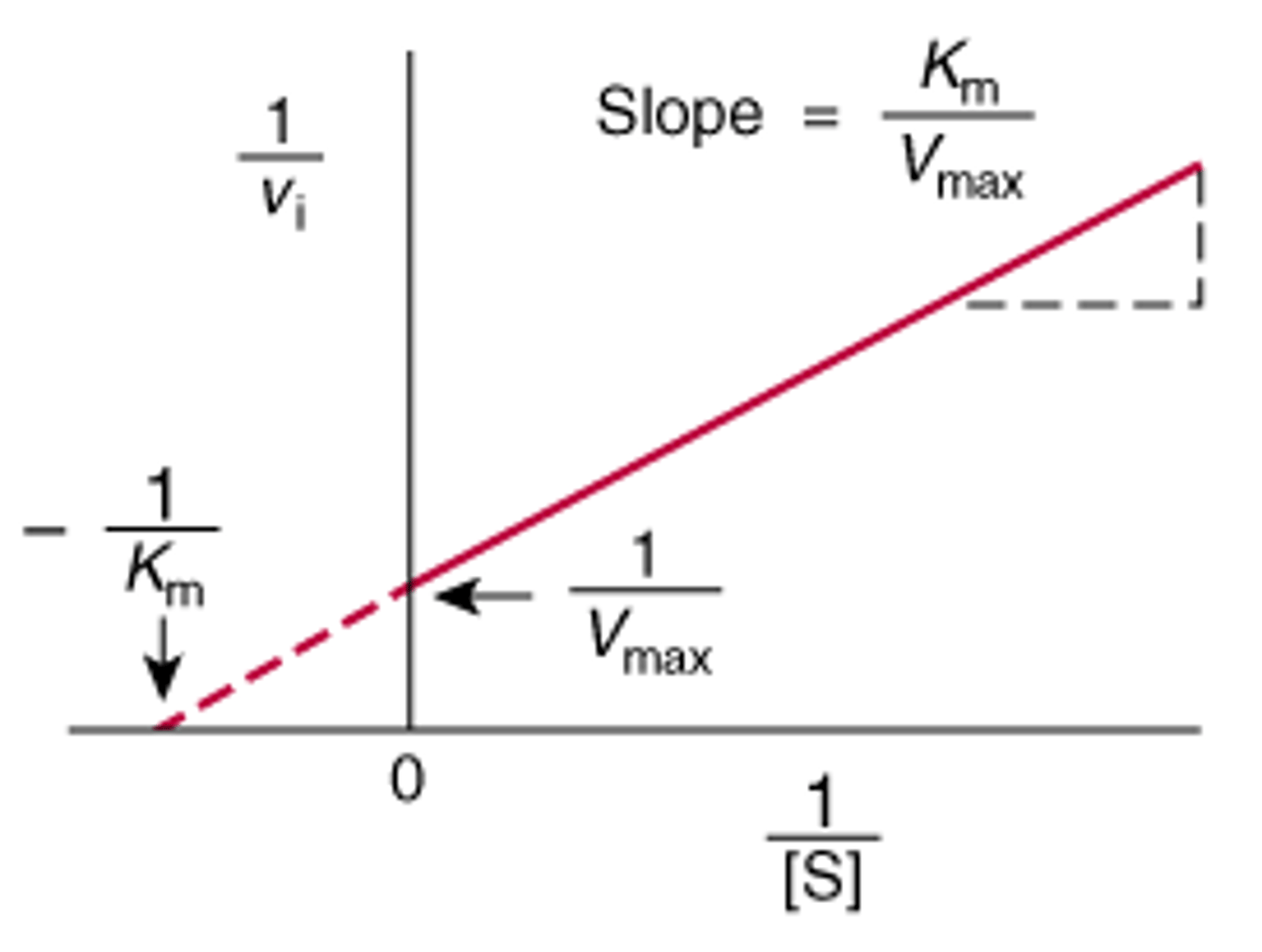
how is Km and Vmax calculated from this
-Km and Vmax for the enzyme are calculated from the negative reciprocal of the x-intercept and the reciprocal of the y-intercept
biochemical pathways between organisms
-many of the central biochemical pathways are very similar in many organisms even though millions of years have elapsed since they last shared a common evolutionary ancestor.
-as a result, very different organisms utilize similar enzymes to catalyze these shared biochemical reactions
enzyme that is found throughout nature
-beta-galactosidase
beta-galactosidase
catalyze the hydrolysis of the disaccharide lactose into the monomers glucose and galactose.
what is beta-galactosidase called in humans
lactase
deficiency in lactase
leads to the conditions known as lactose intolerance.
-beta-galactosidase E. Coli
-historical interest to study
-important for its production in response to cues in its environment which was responsible for our original understanding of how organisms are able to make the right proteins at the right time to maximize their chances of survival.
industrial application of beta-galactosidases
-generation of milk products that individuals who lack lactose can consume without discomfort as the addition of the enzyme breaks the lactose that is naturally present into its monosaccharide compoentns which are more easily digested.
are enzymes identical
although the same type of enzyme in different organisms will catalyze the same reaction, the enzymes themselves are rarely identical.
how do enzyme differ
-they differ in their amino acid sequence and also in the higher levels of their protein organization.
what is the result of differences in enzymes
since structure and function are so closely intertwined, differences in structure will result in functional differences which can be measured.
-for example, although all the beta-galactosidase will hydrolyze lactose, their kinetic parameters such as Km and Vmax will likely differ for different enzymes from different sources.
in the lab, what do we measure Km and Vmax for
-2 different beta-galactosidases
1. obtained from the fungus Aspergillus oryzae
2. from E. coli
what is the natural substrate for beta-galactosidases
lactose
why do we use an artificial substrate
-since the hydrolysis of lactose cannot be followed directly because there are no easily discernable differences in the spectra properties of the substrate and the reaction products, the activity of the enzyme will be monitored using an artificial substrate,
what artifical substrate do we use in the lab
2-nitrophenyl-beta-D-galatopyranoside (ONPG)
-Chromogenic substrate
what is 2-nitrophenyl-beta-D-galatopyranoside
-2-nitrophenol chromophore bonded to galactose through a beta-glycosidic linkage.
how does 2-nitrophenyl-beta-D-galatopyranoside absorbance change
-2-nitrophenol chromophore (ONP) absorbs light at 420 nm when the glycoside bond is hydrolyzed and ONP is liberated.
-the substrate ONPG absorbs very little light at this wavelength and therefore, one can monitor the reaction by measuring the change in absorbance at 420 nm over time.
enzyme inhibitors
-molecules that slow or halt the activity of enzymes.
investigations using enzyme inhibitors
-investigations using enzyme inhibitors have provided profound insights into protein function and they are valuable tools for defining many metabolic and signaling pathways.
pharmalogical agents
-since enzymes are indispensable for most physiological processes, the selective interference of specific enzymes is the principal mode of action of many modern pharmaceutics.
statins
-class of drug
-used to help control cholesterol levels in human blood serum.
-these drugs work because they are inhibitors of a key enzyme involved in the biosynthesis of cholesterol.
how can studying inhibitors help us
-a close examination of the kinetic effects of different inhibitors can yield a wealth of information about enzyme reaction mechanisms.
2 principal categories of enzyme inhibitors
reversible and irreversible
irreversible inhibitors
-tend to form stable covalent bonds with functional groups of an enzyme that are essential for proper function.
-through the use of various types of irreversible inhibitors, the residues that play key roles in catalysis can be identified.
reversible inhibitors
tend to have more fleeting associations with the enzyme, and depending upon how and where they bind, adversely affect enzyme activity in different ways.
examples of reversible inhibitors
a wide variety of different molecules including simple ions, other small molecules, or even larger biological macromolecules can reversibly inhibit enzyme activity.
classifications of reversible inhibitors
competetive, non-competetive, uncompetetive.
how can you determine which reversible inhibitor is acting
the different forms of reversible inhibitions can be kinetically distinguished by examination of Lineweaver-burke plots of reactions where enzyme has been incubated with substrate under varying concentrations of inhibitor.
-changes to Km or Vmax or both for the enzyme in question can then be used to identify the type of inhibitor
competetive inhibition
inhibitors that bind to the active site of the enzyme.
-they compete with the normal substrate
-competetive inhibitors are often described as resembling the substrate (not always the case).
-rather, they look like the transition state that exists during the catalytic sequence
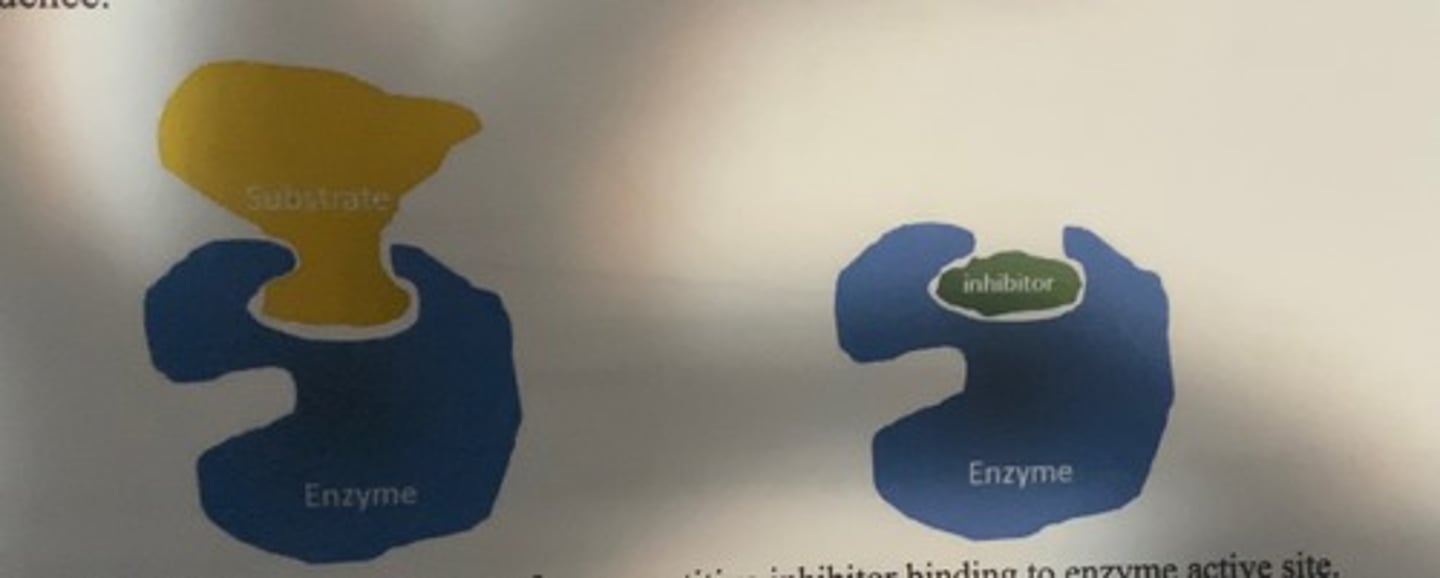
what happens when competetive inhibitor is bound
-when it is in active site, it physically prevents access to the active site by the substrate and no catalysis can take place.
MM plot for competetive inhibition
-as you increase the concentration of the inhibitor, the rate of enzyme activity decreases for every substrate concentration
-at increasingly high enough substrate concentrations, you will reach the maximum rate exhibited by the enzyme when no inhibitor is present (the blue line). this happens because at high concentrations of substrate, the probability of an inhibitor molecule finding its way to the active site approaches zero because there is always a substrate molecule getting there first.
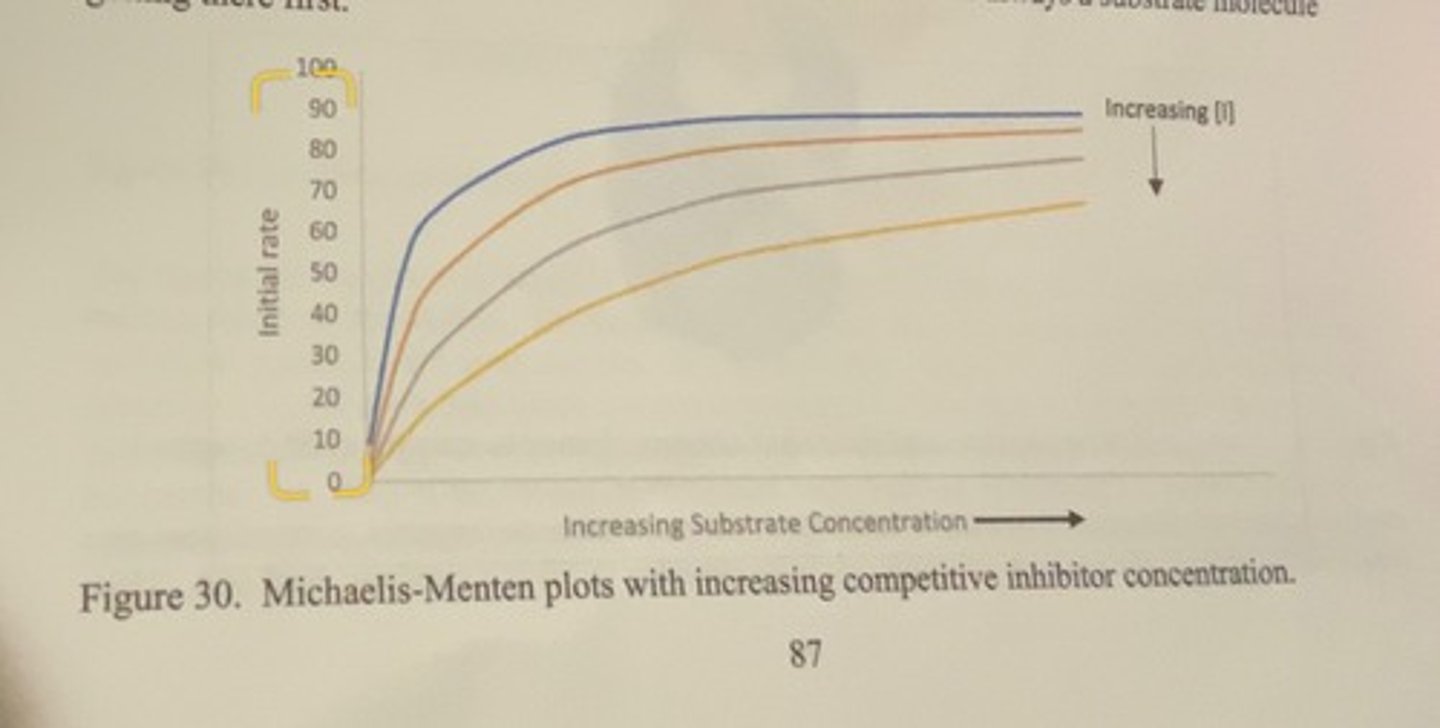
defining features of competetive inhibition
unchanged Vmax but increased Km.
lineweaver burk plot for competetive inhibitor
-same y-intercept (vmax) but the x intercept for the inhibitor is present closer to the origin
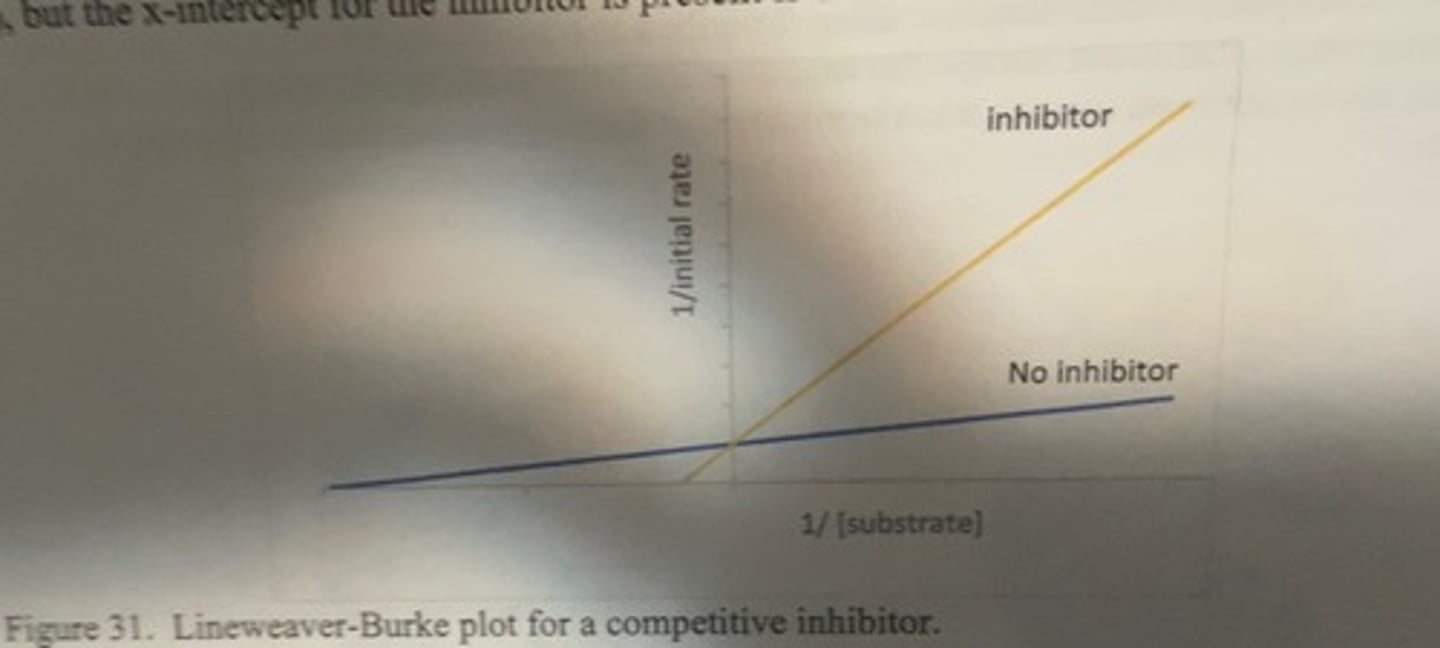
why does Vmax stay the same in competitive inhibition
Competitive inhibitors compete with the substrate at the active site and therefore increase Km (the Michaelis-Menten constant). However, Vmax is unchanged because, with enough substrate concentration, the reaction can still complete
-with enough substrate, can kick out inhibitor to get to Vmax. requires more substrate to get there though so Km increases
Mixed or noncompetitive inhibition
-inhibitors that bind to an enzyme at a site that is not the active site and act independently of the presence or absence of bound substrate
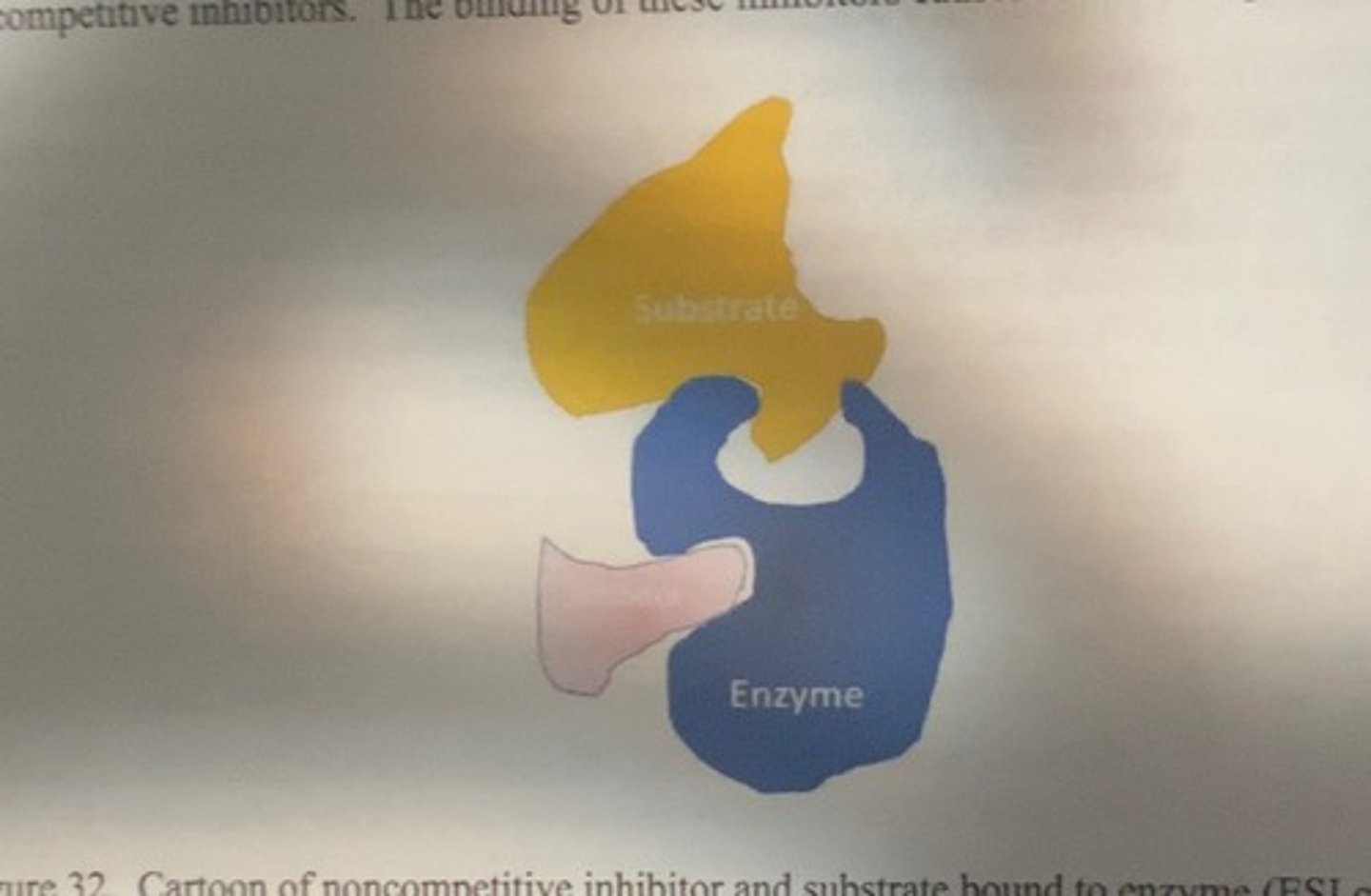
severity of enzyme changes due to noncompetetive inhibition
causes subtle changes to enzyme conformation with the result that even if substrate is able to bind to the enzyme, catalysis can no longer occur because the enzyme substrate inhibitor complex (ESI) is a catalytic dead end
ESI
enzyme substrate inhibitor complex
why does this happen?
probably happens because particular amino acid residues in the active site no longer make the required interactions with the substrate as it proceeds along the catalytic sequence
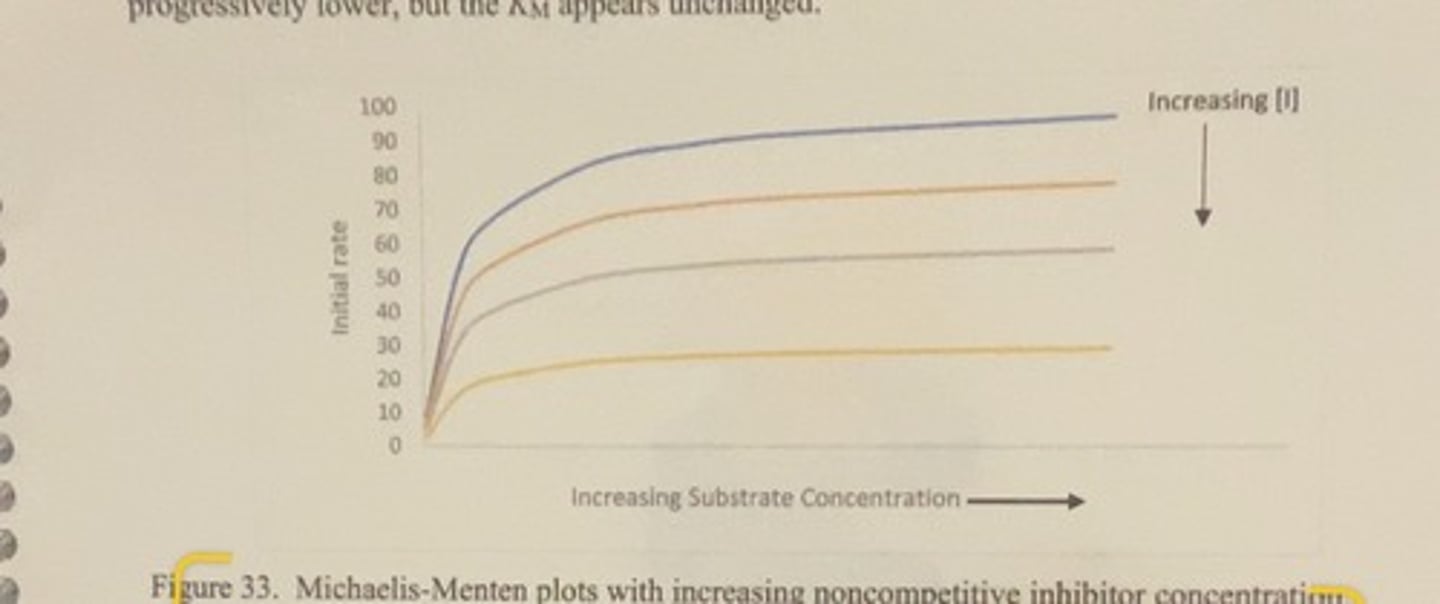
MM plot of increasing noncompetetive inhibitor concentration
-as the concentration of inhibitor is increased, the Vmax gets progressively lower but the Km remains unchanged
Lineweaver Burk plot for noncompetetive inhibitor
-y intercept is higher (lower Vmax) but x-intercept (meaning Km) is unchanged
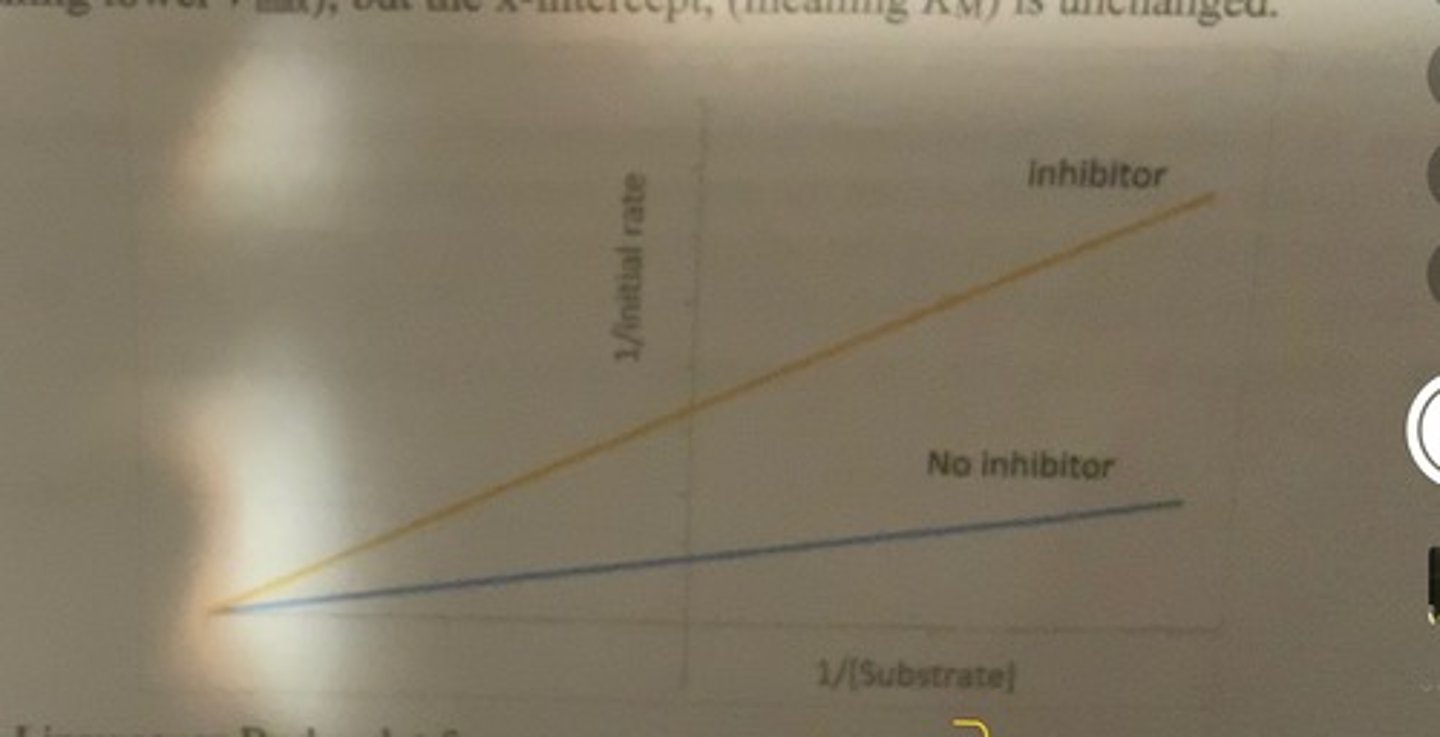
reason
-when inhibitor is present, it will bind to the enzyme making catalysis impossible.
-there are fewer catalytically competent molecules present in the assay cuvette.
-a reduction in the number of enzyme molecules that are capable of catalysis results in lower measured rates at all substrate concentrations.
-this effect cannot be competed away because increasing the concentration of substrate does not change how many enzyme molecules have been rendered incompetent by inhibitor binding.
what is measured rate dependent on
how much enzyme is present
why is Km unchanged
because those molecules which do not have inhibitor bound to them function normally.
-by increasing substrate, won't be able to make a difference
uncompetetive inhibitor
-inhibitors that can only bind to the enzyme after substrate is bound.
-the binding of these inhibitors is probably highly variable and depends upon a conformational change in the enzyme that occurs when substrate is bound that creates a new binding site for the inhibitor.
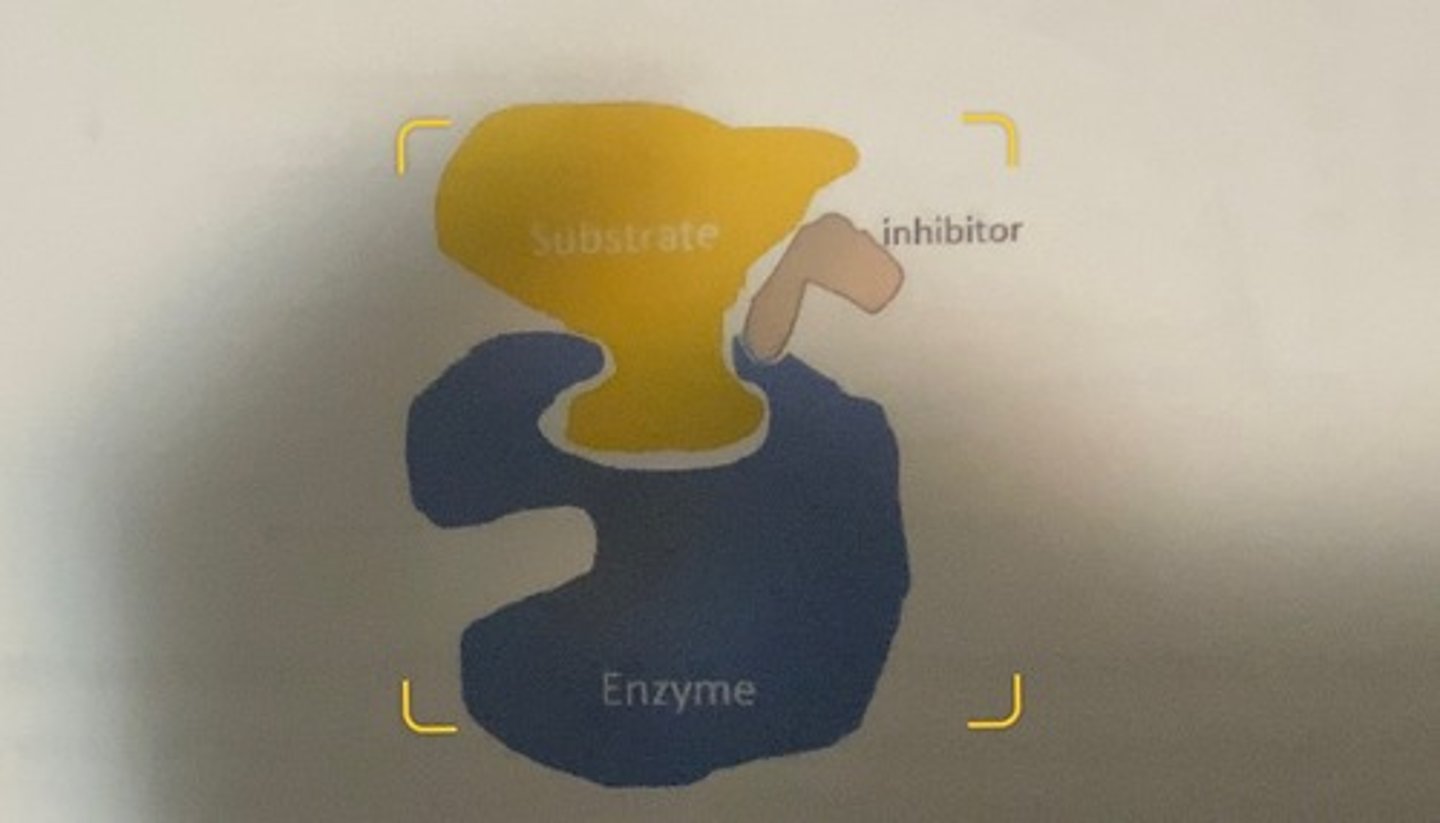
what happens when inhibitor binds
-in some cases, the binding of the substrate to the enzyme active site causes the uncompetetive inhibitor to bind the substrate rather than the enzyme, but regardless of the actual mechanism, the key is that the inhibitor binding event can only take place after substrate binding to the enzyme.
-the ESI complex is a catalytic dead end
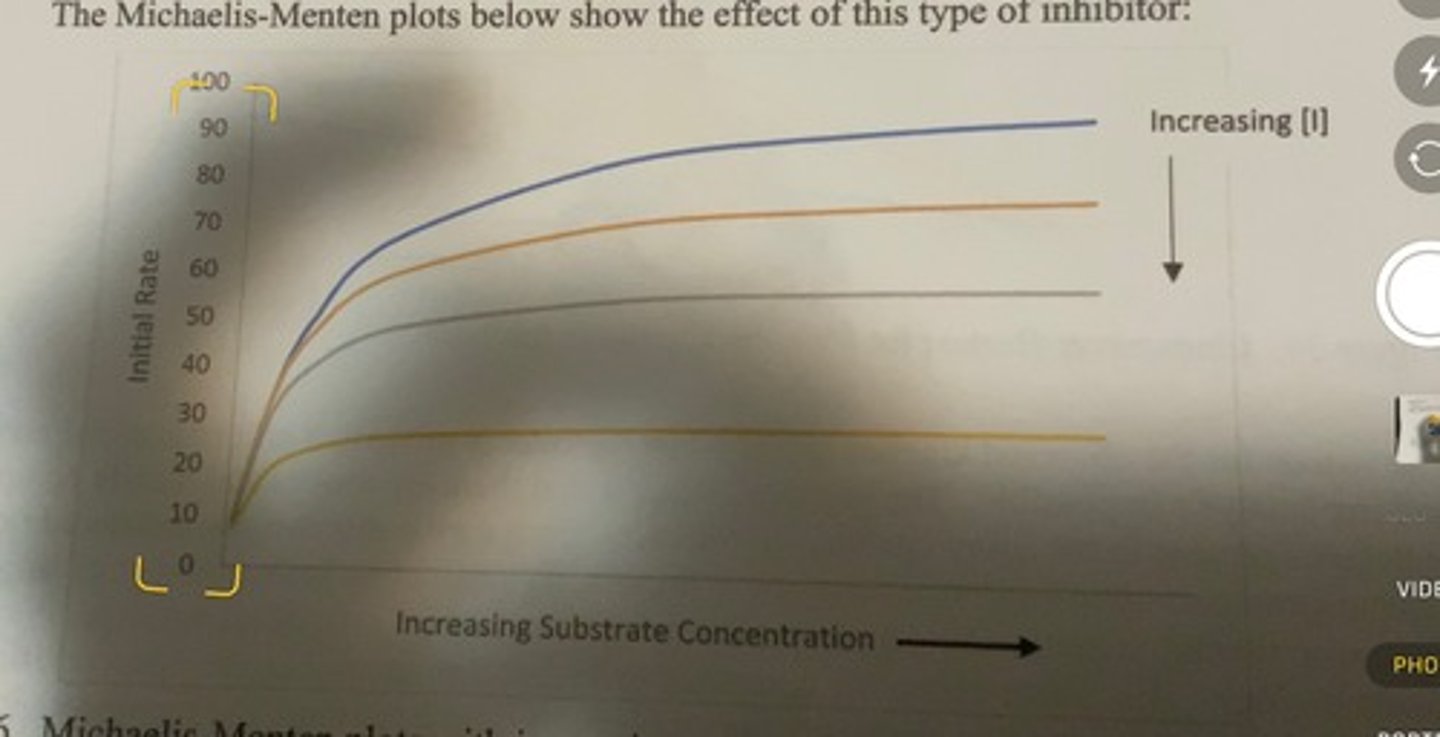
MM plot with increasing uncompetetive inhibitor concentration
as the concentration of inhibitor is increased, the catalytic rate for any particular substrate concentration is lower than when inhibitor is absent
-no amount of substrate can compete away the effect of the inhibitor.
-the inhibitor lowers the actual number of catalytically capable enzymes that are present.
Lineweaver Burk plot for an uncompetetive inhibitor
-Vmax is lower
-lower Km
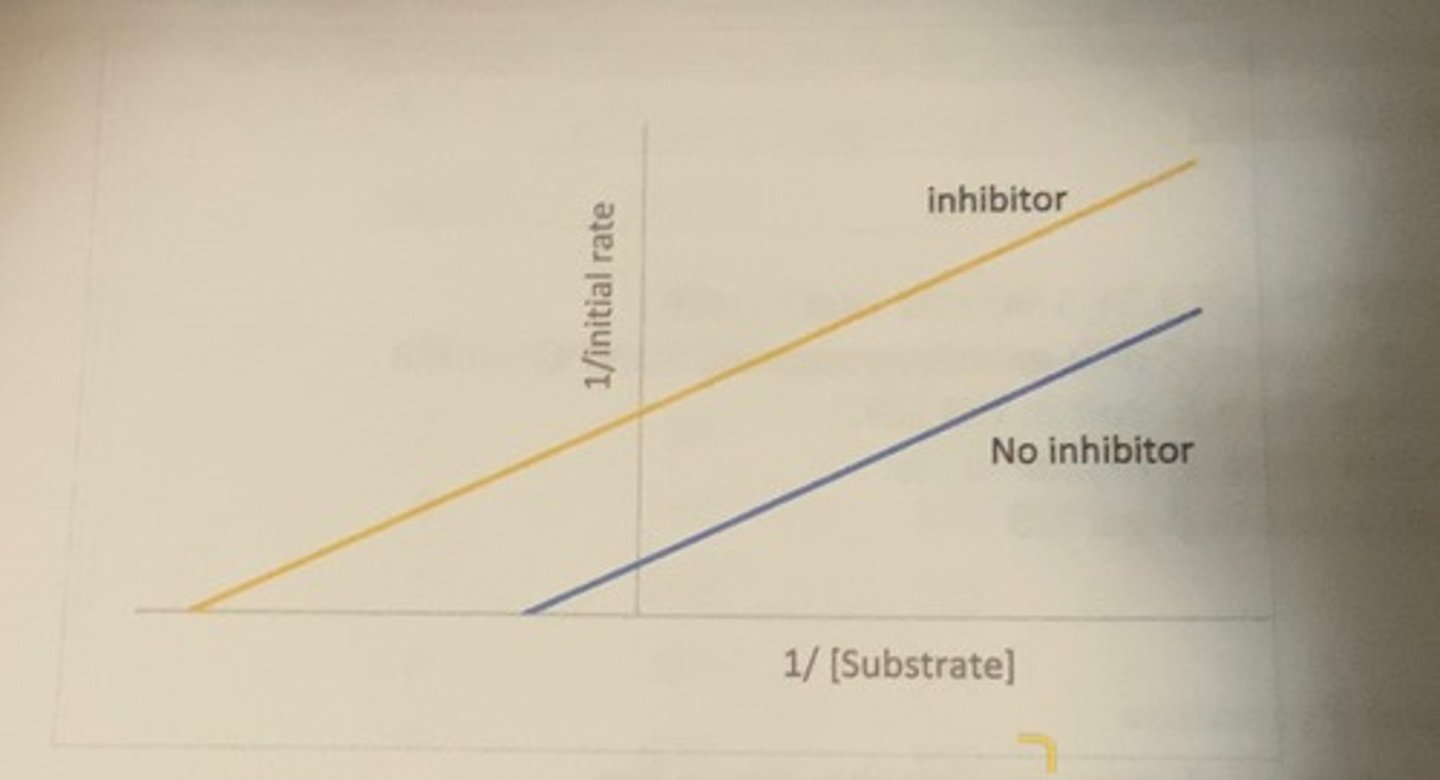
why is Km lower
the inhibitor binding lowers the concentration of ES which disturbs the equilibrium between E and ES with the result that more substrate binds to the available E creating the appearance that the affinity of the enzyme for the substrate (Km) is higher.
in lab, what do we measure the rate of hydrolysis of
ONPG by the enzyme beta-galactosidase
-we use 2 different sources of ONPG at different concentrations
A oryzae
we examined the effect of galactose upon the activity of the beta-galactosidase from A. oryzae
galactose
inhibitor of beta-galactosidase