HAD 381 - Aerobic Gram-Positive Rods
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
Spore forming bacilli
Bacillus spp
Non-spore forming bacilli
Morphologically regular
Listeria, Erysipelothrix, Lactobacillus, Kurthia
Coryneform or irregular
Corynebacterium (from Green koryn club)
Nocardioforms (filamentous) and aerobic actinomycetes
Nocardia, Streptomyces
Spore forming gram-positive rods: Bacillus spp.: Most pathogenic:
B. anthracis (anthrax)
Spore-forming gram-positive rods: Bacillus spp.: Most frequently isolated in CML:
Many species found as contaminant
B. cereus: “Fried rice syndrome”
Emetic toxin
B. subtilis: opportunistic infection
How does anthrax work:
Anthrax spores are inhaled
Anthrax spores enter lungs and travel to alveolar spaces
Spores are transported through the lymph system to the mediastinal lymph nodes where they make toxins that are deadly
The Anthrax Cycle:
Vegetative Forms: Bacteria in animal waste and decomposition (Cattle: Inactive, organism cannot replicate)
When exposed to oxygen, will become anthrax spores — Converts inactive form to active
Can be cutaneous from open wounds/abrasions or infected animal (ex. fly)
Can be inhaled through respiratory complications
Can be ingested
Anthrax:
Primarily a disease of cattle, sheep, horses, and goats
Humans become infected incidentally when brought into contact with diseased animals, which includes their flesh, bones, hides, hair and excretement
BIOTERRORISM AGENT
Cutaneous anthrax:
Most common acquired via injured skin or mucous membranes (95%)
Gastrointestinal anthrax:
Analogous to cutaneous anthrax but occurs on the intestinal mucosa
Inhalation anthrax:
Wool sorters’ disease
Pulmonary, rare
Workers who handle animal hide are more susceptible
Anthrax Toxin Subunits:
2 A subunits
1 B subunit
All together create “holotoxin” which apparently random mixtures of A subunits
A subunits:
Edema factor (adenylate cyclase)
Lethal factor protease
Edema factor (adenylate cyclase):
Converts ATP → cAMP which causes activity of cell such as imbalance of fluid, which causes accumulation of fluid
Lethal factor protease:
Receptor attaches to cell and will kill macrophage to survive
B subunit:
Protective antigen
Anthrax Virulence:
poly-D-glutamic acid capsule
tripartite toxin
Protective antigen (PA) : B subunit
Edema factor (EF) : A subunit
Lethal factor (LF) : A subunit
The three proteins combine in pairs to produce the lethal factor (PA+LF) ad edema factor (PA + EF)
Bacillus spp. BAP
Aerobic or faculative anaerobic
Large (4-5 mm), flat, spreading, grey white colonies, with irregular margins
Many beta hemolytic (Except B. anthracis which is non hemolytic)
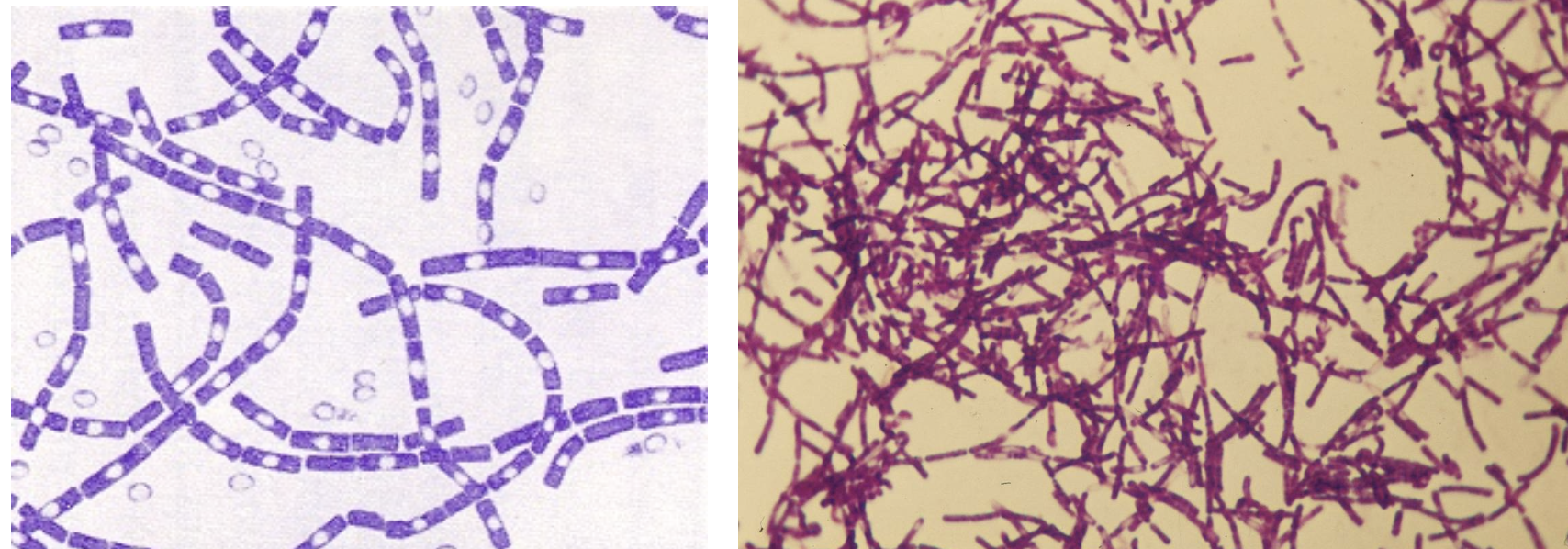
Gram stain of Bacillus anthracis, showing endospores
Large rectangular rods, form chains
Spores do not cause swelling of cells and perfectly fit inside
How to differentiate from Bacillus spp. gram stain/microscopic wise?
Malachite green/safranin spore staining
Malachite green stains spore green
Safranin stains rods
Differential tests for B. anthracis
Hemolysis negative
Motility negative
Gelatinase negative
Salicin fermentation negative
Penicillin susceptible
Other Bacillus spp. are positive and resistant (except B. subtilis which is susceptible to penicillin)
Bacillus cereus clinical significance:
Associated with gastrointestinal infections, local infections, and systemic infections
Specimens for isolation
Blood and CSF (systemic infection)
Sputum and Pleural Fluid (pneumonia)
Wound, eye, bone marrow, joint fluid (Local infections)
Feces and suspect food (foodborne infections)
Submitted to public for analysis
Spore-forming rods:
Aerotolerant Clostridium
Clostridium tertium and Clostridium perfringens are examples
Grow best in anaerobic environment, but may grow in atmosphere with oxygen
General clue is initial sparse growth of tiny colonies compared to the growth on anaerobic media/conditions displaying much more growth and larger colonies
Gram stain morphology resembles Bacillus species (large, with or without spores)
How to differentiate between Bacillus spp. and Clostridium spp.
Most Clostridium are catalase negative and form spores under anaerobic conditions
Bacillus spp. are catalase positive and form spores under aerobic conditions
Species of Corynebacterium other than C. diphtheriae
Listeria, Erysipelothrix, Kurthia are coryneform organisms too
Generally considered contaminants when encountered in cultures of clinical material
Inhabit the skin and mucous membranes of the upper respiratory tract, urethra, and vagina (normal flora)
Erysipelothrix can cause infections in otherwise normal persons (immunocompetent)
Listeria can cause infections in both normal hosts, but mostly immunocompromised persons
Kurthia has been rarely isolated in cases of endocarditis
Diphtheroid infection has been associated with?
Vascular prosthesis or immunosuppression
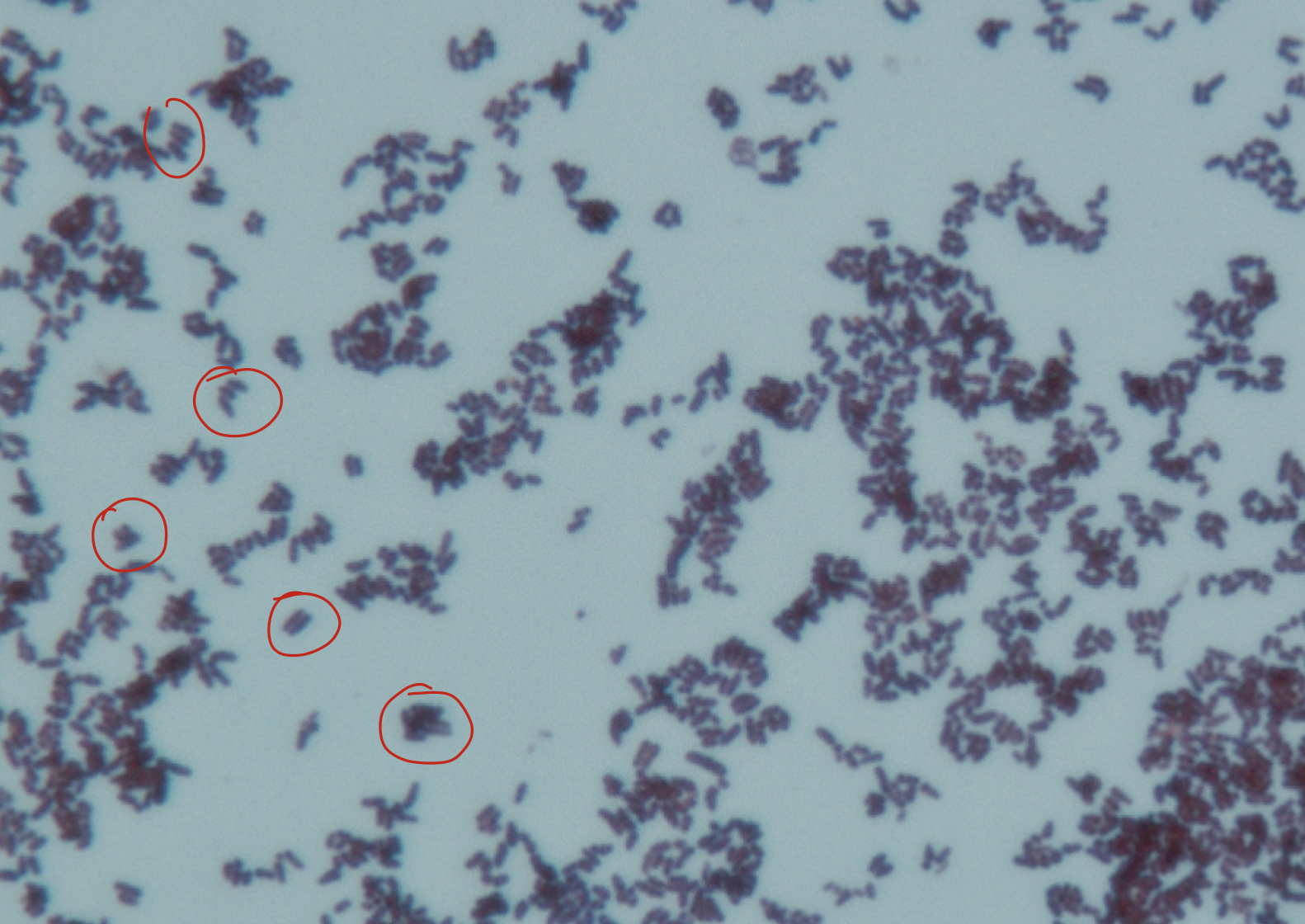
Coryneform or diphteroid bacteria morphology
Irregular shape (pleomorphic)
Arranged in V forms or palisades (non-branching)
“Chinese-letter” or “picket fence” arrangement
Stains unevenly (metachromatic)
This uneven stain may lead to beaded appearance
May need to report as gram variable if staining unevenly
Corynebacterium spp.
Non-spore forming
Non-motile
Catalase positive
Ferment glucose and mannitol
No H2S gas production
Non-acid fast
Corynebacterium diphtheriae
Causes diphtheria
Potentially fatal illness that kills 5% to 10% of infected persons
Affects the mucous membranes of the nose and throat: forms pseudomembrane
Can also affect the skin by infecting existing open wound": cutaneous
In advanced stages, toxins reach circulation and can damage the heart and nervous system: systemic infection
Isolated infrequently in developed countries since vaccination has been implemented, but still a problem in underdeveloped or developing nations
Diphtheria infection in oropharynx
Causes a strong inflammation and formation of pseudomembrane that can lead to respiratory obstruction (suffocation)
Produce a very powerful exotoxin which spreads by the bloodstream affecting heart muscle and nerve endings: can cause paralysis and is systemic
Diptheria toxin and diphtheria toxin receptor:
Toxin gene encoded by a bacteriophage
Toxin is a single polypeptide chain consisting of two subunits linked by disulfide bridges, known as A-B toxin
Binding to the cell surface of the B subunit allows the A subunit to penetrate the host cell and block protein synthesis by transfer of ADP-ribose from NAD to a diphthamide residue of EF-2
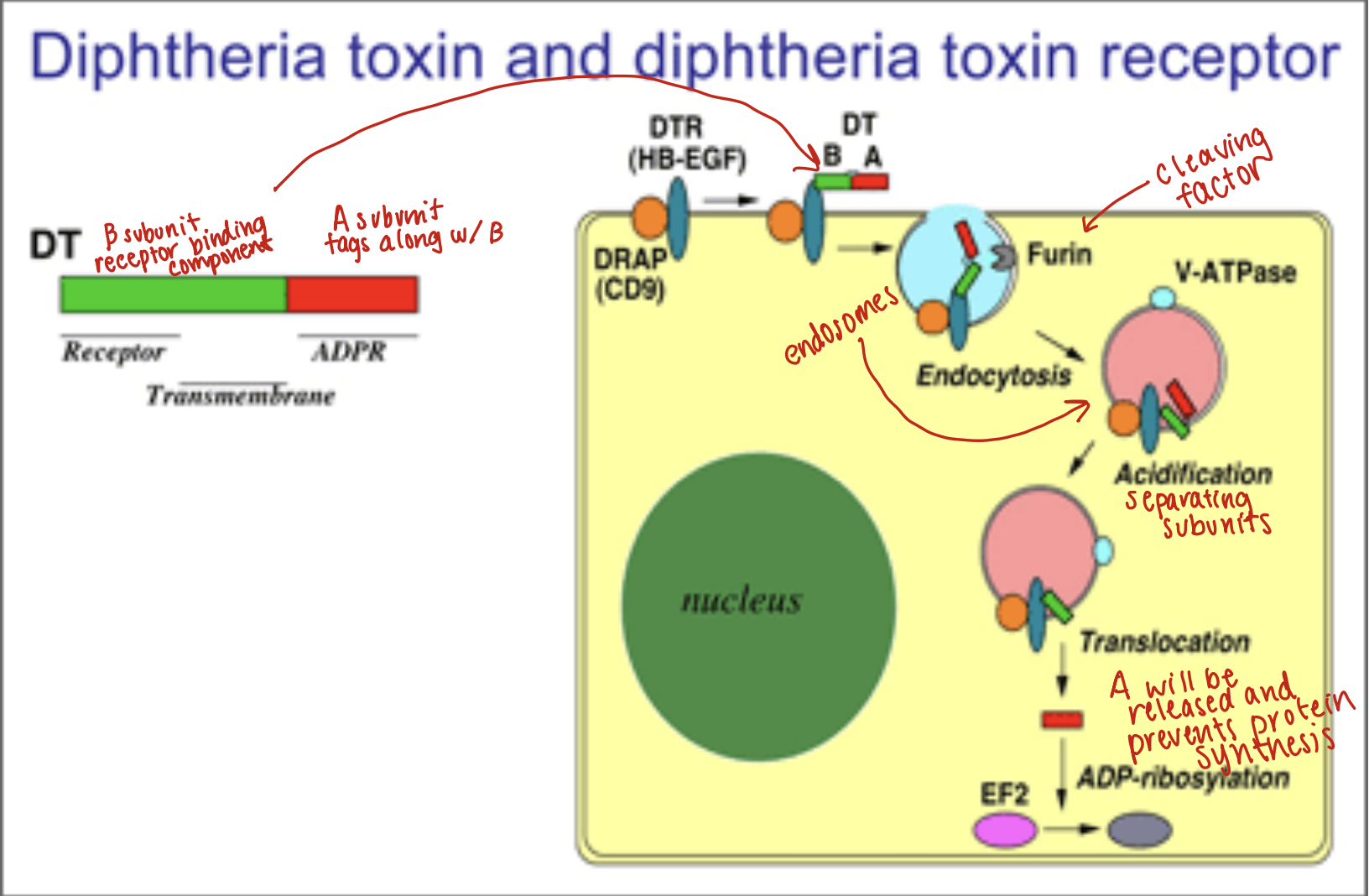
Diphtheria Toxin:
Synthesized by toxigenic strands of Corynebacterium diphtheria
Toxin enters through receptor mediated endocytosis
Acidification of endocytic vesicle allows A to dissociate from B and then enter the cytoplasm
C. diphtheriae macroscopically
Grow in BAP (common medium for nasopharingeal specimens)
Colony type depends on biotype:
Biotype Intermedius: Small gray or translucent
Biotypes mitis, belfanti and gravis: White opaque, larger
Non hemolytic or alpha hemolytic
Grow best at 37 C on a blood or serum containing medium (Loeffler’s serum or serum tellurite medium)
Facultative anaerobes
Can grow in ambient air or may be incubated in a 5-10% CO2-enriched environment
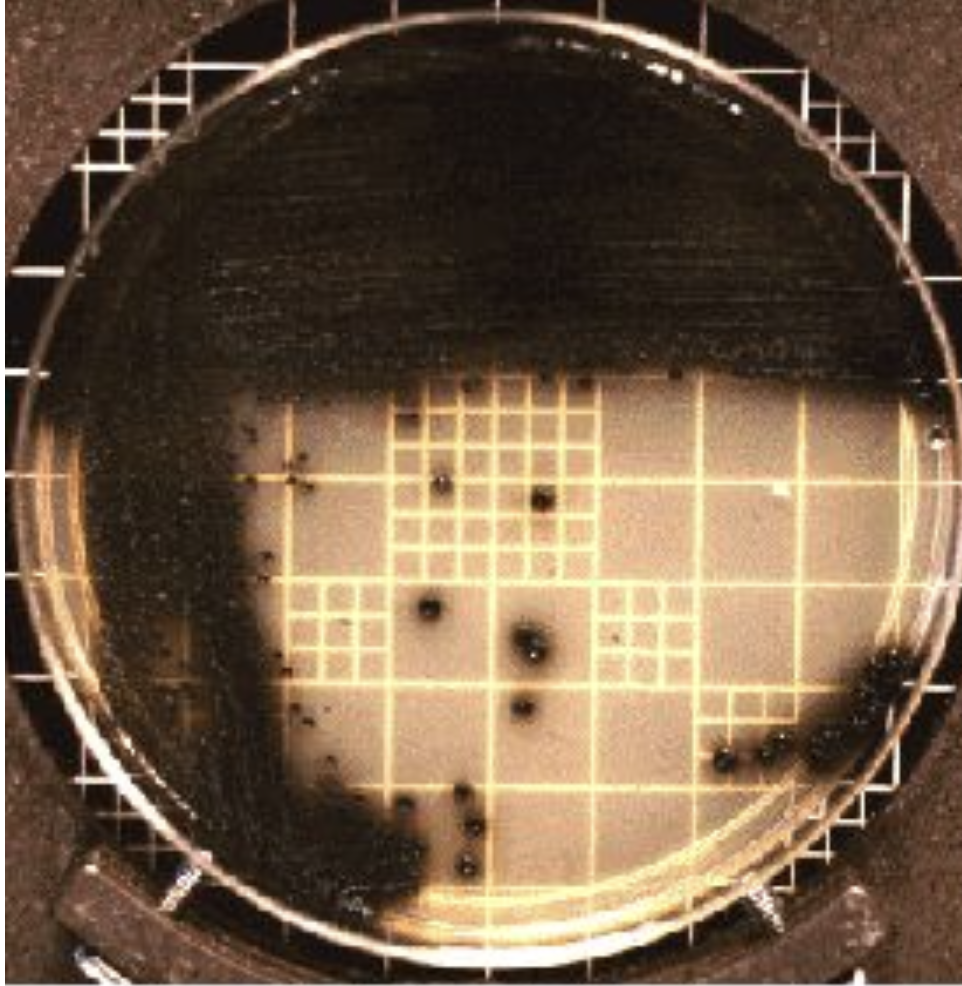
Serum tellurite medium
Also known as Cystine-tellurite or Tinsdale medium
Black colonies indicated tellurite reduction
Selective medium when C. diphtheriae is suspected
Inhibits normal flora from the nasopharingeal tract
Loeffler’s medium:
Used to enhance granule formation before Methylene Blue staining, Alkaline Blue or Albert’s and Neisser’s (Not differential or selective)
Characteristics of Loeffler’s medium:
Volutin (metachromatic) dark purple granules composed of inorganic polyphosphates (volutin) giving the rods a beaded appearance when stained with Neisser or Albert stains
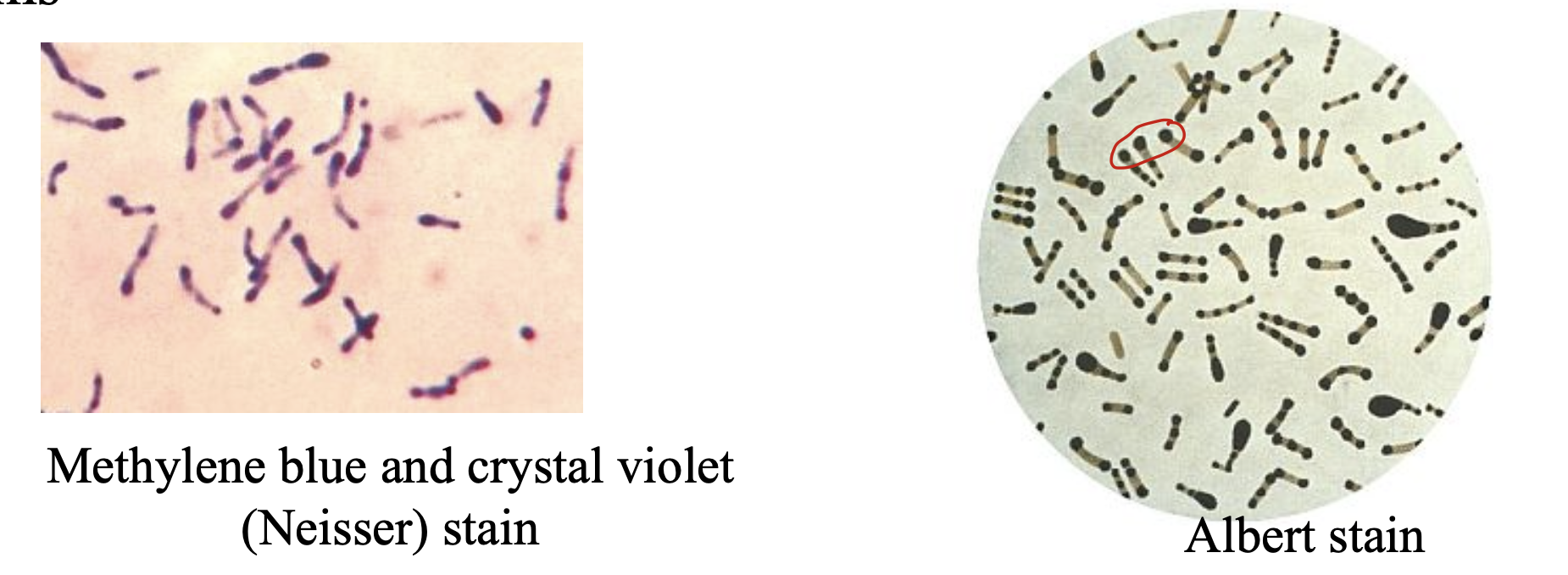
C. urealyticum
Causes UTI
Multi-drug resistant
Lipophillic
Urea positive
Urinalysis may reveal alkaline urine and struvite crystals (Presence of struvite crystals) magnesium ammonium phosphate due to urease activity
Hold urine cultures more then 24 hours to obtain isolation - slow growing
C. jeikenium
Multidrug resistant — can be fatal, esp to immunocompromised
Cause of endocarditis, septicemia, foreign body infection
C. pseudodiphtherium
Normal flora
Can cause pneumonia
C. equi
Now Rhodococcus equi
Found in soil, causes diseases in horses and goats
In immunocompromised cause TB-like infections (mycobacterium)
Arcanobacterium general characteristics
Short pleomorphic GPR
Catalase negative
A. pyogenes and A. haemolyticus
Beta-hemolytic (narrow)
May be PYR positive
A. haemolyticum
CAMP inhibition positive
Xylose and gelatin negative
A. pyogenes and A. bernardiae
CAMP inhibition negative
Xylose and gelatin positive
A. pyogenes
ONPG positive
Regular rods:
Non-spore forming, gram positive rods which have two parallel sides with rounded ends
Includes Listeria spp. Lactobacillus, and Erysipelothrix
Colony morphology similar to other microorganisms, so the gram stain is important role in their identification
Listeria spp.
Non-spore forming, Gram positive bacilli
Facultative intracellular anaerobe
Catalase positive and oxidase negative
Colony and Gram stain morphology may resemble Group B streptococci
Main pathogenic species of Listeria
Listeria monocytogenes
Food poisoning (listeriosis)
Can cause outbreaks invasive listeriosis
Affects pregnant women, their babies (neonatal meningitis), the elderly and immunosuppressed adults
Other rare human pathogen of listeria:
L. ivanovii
Listeria monocytogenes virulence factors:
Intracellular pathogen
Replicates in pathogen
Actin binding protein (movement inside cytoplasm)
Phospholipase C and Listeriolysin O (lysis of the vacuole)
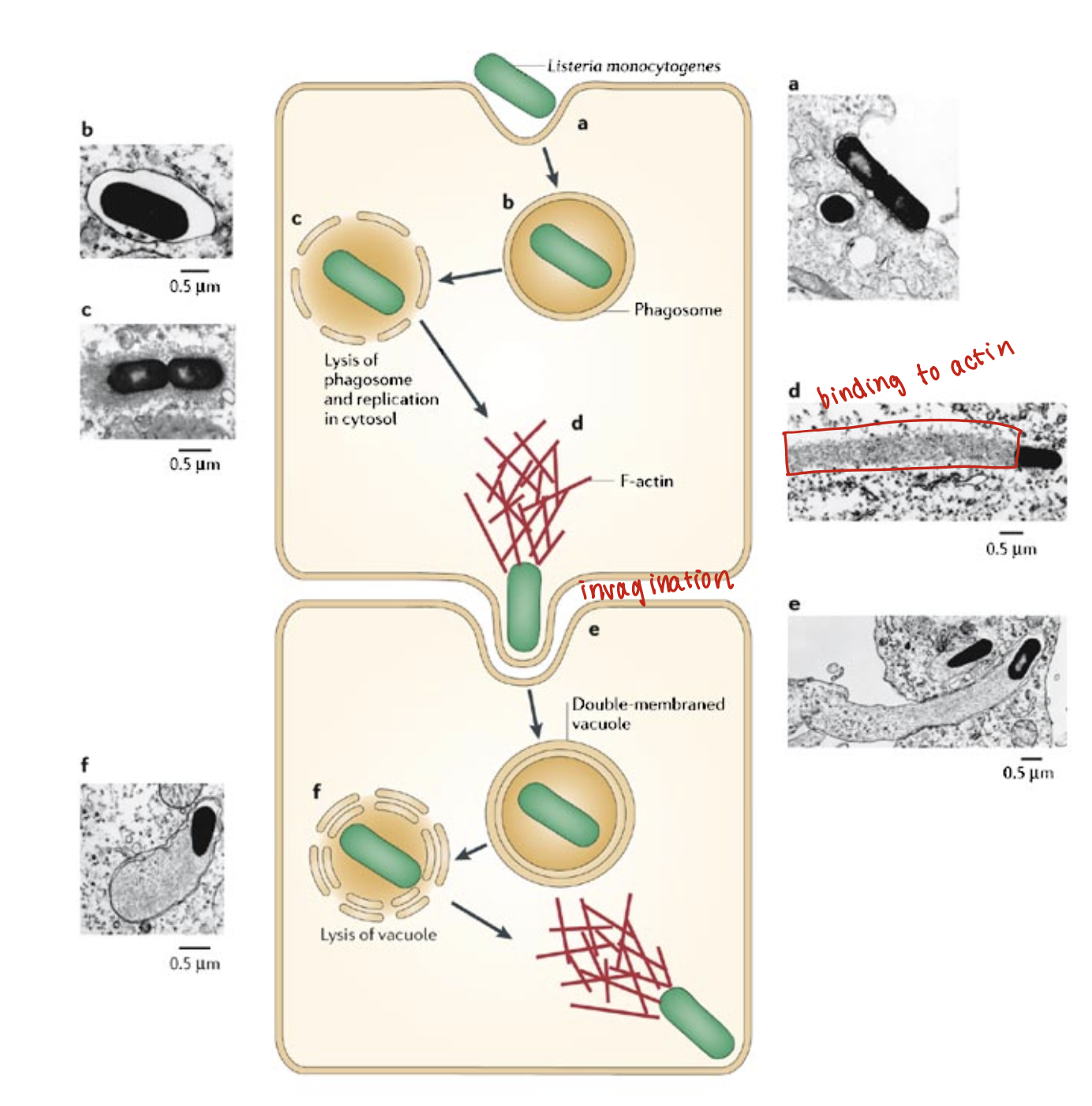
Listeria Monocytogenes laboratory tests
Grow well on a wide variety of laboratory media
Optimal growth 30-35C
Grow well at 4C (selective condition)
Beta hemolytic (other Listeria spp are non-hemolytic — differential)
Catalase positive (Strep is negative)
Listeria monocytogenes macroscopically
Small
Beta hemolytic
May have blue/green sheen
Narrow zone of beta-hemolysis
Resembles GBS and may react with Streptococcal Group B and G antisera
CAMP positive
L. monocytogenes microscopically
Coccobacilli
Single or short chains
No branching
Can form filament in older cultures
Can be mistaken by streptococci (GBS also cause neonatal meningitis)
Listeria monocytogenes motility:
Non motile at 35C
Motile at 25C "
“Umbrella” type or Xmas tree shape
Tumbling motility (end-over-end) in hanging drop preparations
Hanging drop done with concave microscope slide and you put drop of organism on coverslip and put the the overslip on top, upside down
Important L. monocytogenes: ID tests
CAMP test positive (GBS also CAMP+)
Non motile at 35 C (like GBS) motile @25C
Ferments glucose, trehalose, salicin
Esculin hydrolysis: positive
Used in differential media, black colonies
Erysipelothrix spp.
E. rhusiopathiae causes economically important disease in swine called erysipelas (different from erysipelas that is caused by GAS) also in lambs, calves, birds, and fish
Humans become infected (rare) through exposure to infected or contaminated animal or animal products
Occupational disease: affects veterinarians, abattoir workers and fisherman (“fish handlers’ disease”) form of skin infection in the fingers (eryispeloid) seal finger or whale finger
Occasionally cause septicemia and endocarditis
E. rhusiopathiae
Non sporing gram positive bacilli or long branching filaments
Might look like gram-negative (decolorize easily)
Erysipelothrix rhusiopathie macroscopically/lab characteristics
Non-motile
Facultative anaerobe
Improved growth @ 5-10 % CO2
On BAP two distinct colony forms (non-hemolytic)
Smooth: small convex and circular, transparent glistening colonies
Rough: large, flat opaque
Erysipelothrix rhusiopathie ID tests
Catalase neg
Oxidase neg
Indole neg
Urea neg
Esculin neg
Motility neg
TSI K/A, with H2S
(as opposed to L.monocytogenes)
Weakly fermentative (glucose +)
Gelatin stab at 25C (Not used for gelatinase, but growth pattern)
Growth": pipe cleaner or test tube brush pattern (Gelatinase -)
Lactobacillus
Normal microbiota of vagina, gastrointestinal tract, and oropharynx
Widely distributed in nature
Make up 90% of the normal human vaginal flora
Also normal flora of mouth and GI tract
Facultative anaerobe
Part of the lactic acid bacteria group
Convert lactose into lactic acid
Opportunistic infections
Bacteremia, endocarditis, meningitis (rare)
Regulators of the vaginal ecosystem
Lactobacillus
May prevent urogenital infections by
Production of lactic acid, bacteriolicins (toxins produced by bacteria to inhibit growth of other bacteria), and hydrogen peroxide
Competitively excluding attachment and vaginal colonization by other microorganisms
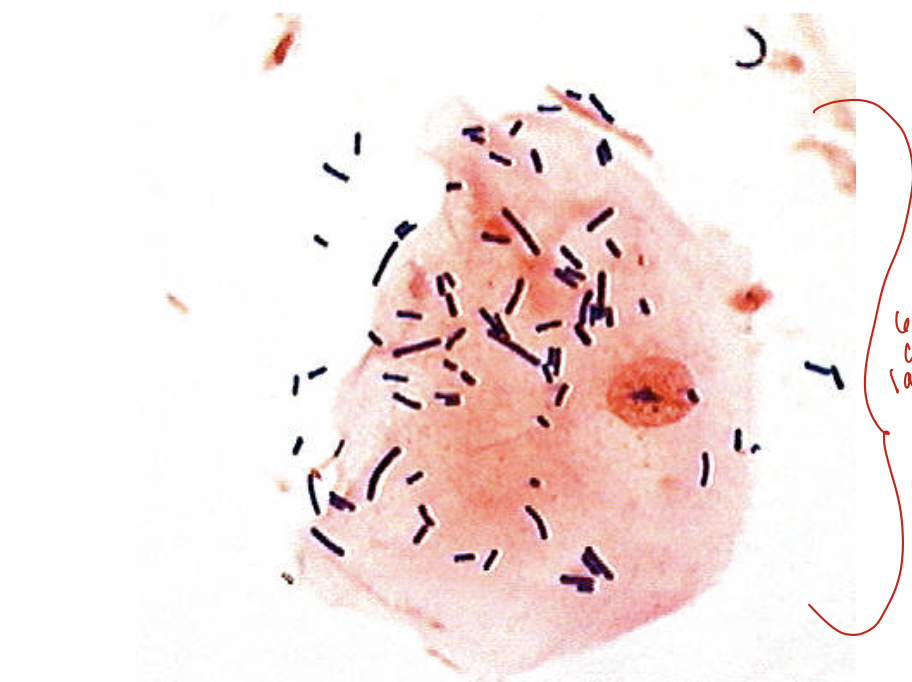
Lactobacillus sp. adhering to a vaginal epithelial cell as seen on a Gram stain of vaginal fluid (x 1,000 magnification)
60-70% covered in lactobacillus, which is normal
No lactobacillus, could be indicitive of BV
Coccobacilli also indicative of BV
Lactobacillus spp. microscopic morphology
Long, slender gram positive pleomorphic bacilli
Non-spore forming
Lactobacillus lab diagnosis results
Catalase negative
Oxidase negative
Nitrate negative
Indole negative
H2S negative
Gelatinase negative
Kurthia spp.
Associated with endocarditis, pneumonia, and bacteremia (patient must be seriously immunocompromised to acquire)
Regular, unbranched, relatively large GPB
Rounded ends, occur in chains
Motile by peritrichous flagella
Non-acid fast
Catalase positive
Oxidase negative
Irregular Rods
Gram positive rods which share pleomorphic microscopic morphology
Gardnerella
Aerotolerant Actinomyces
Actinomycetes
Nocardia
Gardnerella
Best medium for growth is human blood bilayertween (HBT) agar; V agar with human blood is also used for isolation
Beta hemolytic on HBT (Bc lots of normal flora in vaginal environment)
Capnophilic
Inhibited by Sodium polyanethole Sulfonate (SPS) → anticoagulant that prevents immune cells from attacking organism that are present
Gardnerella clinical significance
Normal flora
Loss of lactobacillus and predominance of Gardnerella and anaerobic GNR are associated with bacterial vaginosis
Linked to maternal and neonatal infections
Gardnerela vaginalis
A corynebacterium-related species (previously C. vaginalis)
Causes bacterial vaginosis (BV)
Different from vaginitis (Acquired from candida)
Typically associated with other bacteria
Bacteremia (rarely)
Common in asymptomatic women (70%) and girls (14%)
Also found in male urethra
Gardnerella identification:
Most laboratories presumptively identify using Gram stain, catalase, and colony morphology
Gardnerella microscopic morphology:
Gram-variable rod: Could stain as gram negative or gram positive because peptidoglycan layer is much thinner than the Corynebacterium, Lactobacillus and Staphylococcus
Gardnerella Colony morphology:
Tiny, white to gray colonies
Non-hemolytic on BAP
Appears after 48-72 hours of incubation
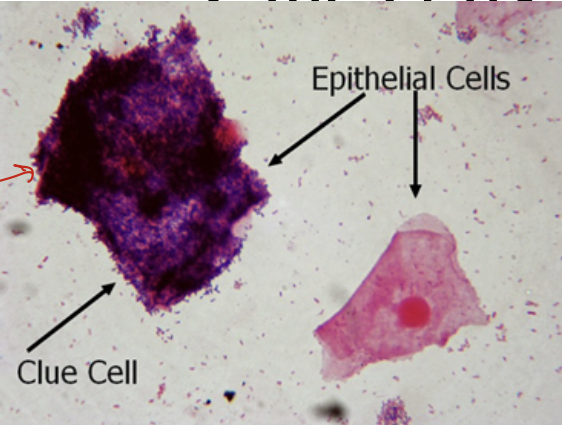
Bacterial Vaginosis: Clue cells
Many labs use Gram stain to evaluate the presence or absence of bacterial vaginosis
In these smears, vaginal epithelial “clue cells” will be covered with bacterial morphotypes. Sheer number of organism cover the margin of epithelial cells
Gardnerella Key Biochemical Reactions:
Hippurate and starch positive
Alpha-glycosidase positive and beta-glucosidase negative (enzymes present in cleavage of diff bonds present in carbohydrates)
Raffinose negative (carbohydrate)
Inhibited by SPS
Whiff test:
A drop of 10% KOH mixed with vaginal fluid and production of fishy smell of indicative of positive whiff tests and bacteria vaginosis
Gardnerella vaginalis susceptibily
Metronidazol, triemetroprim ad sulfonamide
Differentiates from other catalase negative, coccobacillus (e.e. lactobacillus)
Nocardioforms (forms in brain tissues) and aerobic actinobacteria (previously known as Actinomycetes)
Fungus like bacteria
Aerobic, facultative anaerobes or obligate anaerobes
Branched
Filamentous hypahe
Reproduction
By producing spore
By fragmentation of the hyphae
Aerotolerant Actinomyces general characteristics
Mykes Greek for “fungus”
Exhibit true branching
Non spore-forming
Non-acid-fast
Facultative anaerobes
Capnophilic
Grows best anaerobically
Aerotolerant Actinomyces Identification: Microscopic morphology
Irregular staining, pleomorphic, GPR
Diphterodial and branching rods common
Microscopy smears may show filaments with branching
Aerotolerant Actinomyces macroscopically
Microcolonies form after 18 to 24 hours incubation
“spider” or granular centered colony with peripheral branching filaments
Some form colonies that are smooth without branching filaments
Abscess drainage may contain visible yellow to orange sulfur-like granular colonies
Aerotolerant Actinomyces Key biochemical reactions
Most are catalase negative
Non motile
Fermenters
H2S positive
Gelatin negative (straight line growth)
Additional biochemical testing (traditional methodology or rapid/commercial kits) can be performed if speciation is necessary including testing
Other rare human pathogen of Listeria:
L. ivanovii
Aerotolerant Actinomyces clinical significance:
members of the commensal or normal flora in various
body sites, especially the mouth.
They are the causative agent of actinomycosis, which causes abscesses, often of the jaw, which drain via sinus
tracts to the skin surface. Lumpy jaw
Aerobic Actinomycetes general characteristics:
Group of GPR that form thin, beaded, branching filaments.
Some extend filaments into the air to form aerial mycelium
Some are partially acid-fast
May form microcolonies
Infrequent isolates
Aerobic Actinomycetes useful biochemical tests:
Lysozyme resistance
Substrate decomposition of Casein, Xanthine, and Tyrosine
Opacification of middlebrook 7H10 agar
Gelatin liquefaction
Acetamide
Arylsulfatase
Citrate
Commercial kits
Nocardia spp general characteristics:
Posses tuberculostearic acid like Mycobacterium spp. Contain short chain of mycolic acid in their cell wall as all the Gram positive. The presence of mycolic acid
is standard in all but the length of the chain varies among genera.
Opportunistic pathogens
Obligate aerobe
Some species require cooler temperature for optimum growth
Most commonly isolated Actinomycete
Branching, beaded, filamentous bacteria
Can cause "Sulfur granules" in nocardial mycetomas
stains acid fast* (weakly)
Mycobacteria strong acid fast
Actinomyces spp.non acid fast
L-forms (cell wall deficient organisms) can survive in macrophages for days, may account for treatment failure
Nocardia microscopic morphology:
GPR
Branching or beaded
Partially acid-fast (Modified Kinyoun stain)
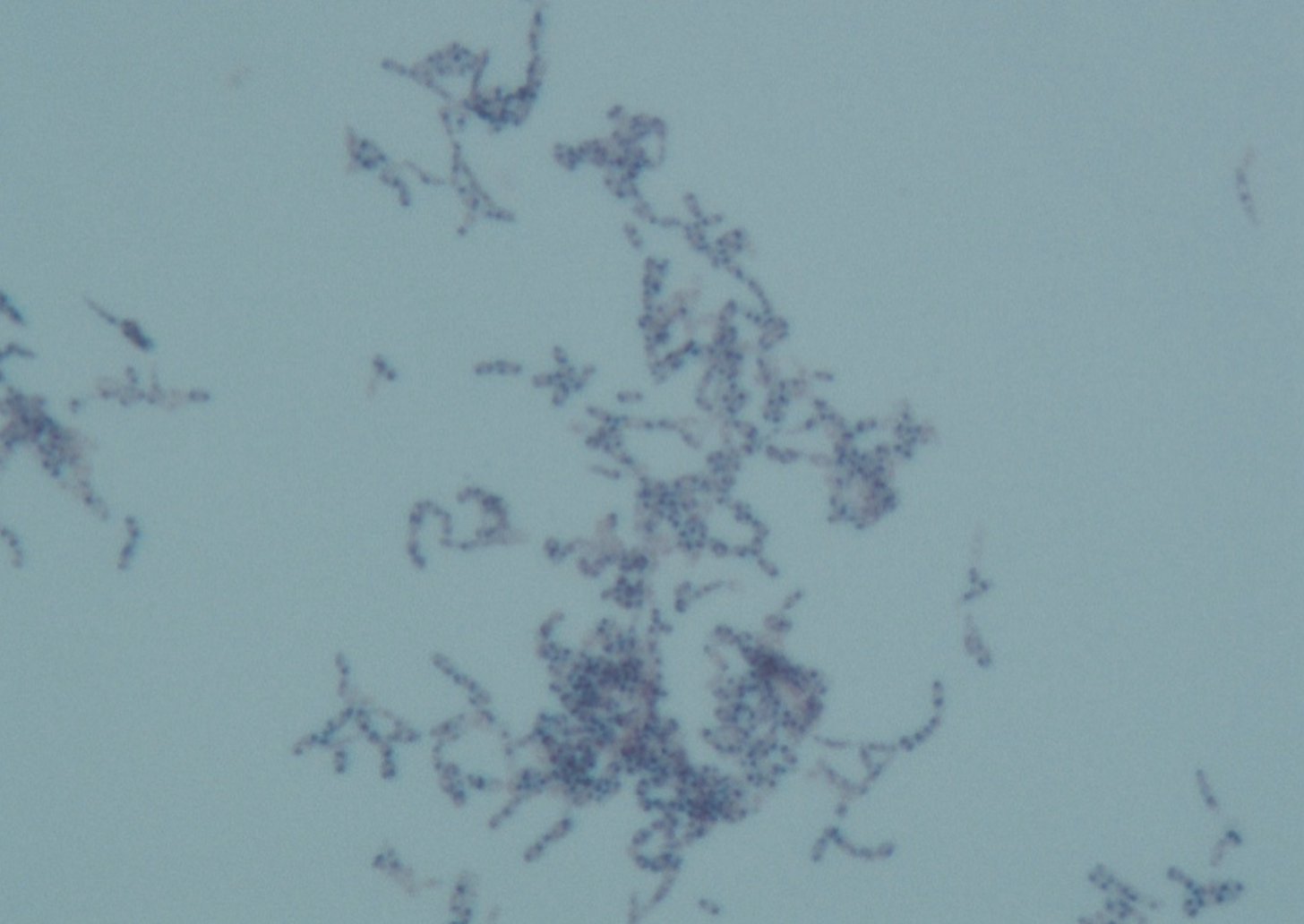
Nocardia colony morphology:
Wrinkled, dry, chalky, adherent
White to yellow, orange, tan, or brown
Beta-hemolytic on SBA
Selective medium used for Legionella can grow Nocardia
Observe aerial mycelium and conidia on slide culture
Nocardia key biochemical reactions:
Speciate with molecular methods
ID to complex using biochemicals and susceptibility results
Catalase positive
Arylsulfatase resistant
Most are Urease positive
Nocardia clinical significance:
An immunocompromised individual inhales the organism into their lungs and develops respiratory infections. From there it can disseminate to other parts of the body and be isolated from blood and cerebrospinal fluid.
80% of lung infections caused by N. asteroides
Immunocompetent persons usually develop skin infections due to traumatic injury
» Mycetoma
» Lymphocutaneous
» Superficial skin infection
80% of skin infections caused by N. brasiliensis
Granules resembling the sulfur granules of Actinomyces can be observed in pus
Can spread from blood to brain, skin, eyes, kidney, joint, bones, and heart
80% of lung infections are caused by?:
N. asteroides
80% of skin infections are caused by?:
N. brasiliensis
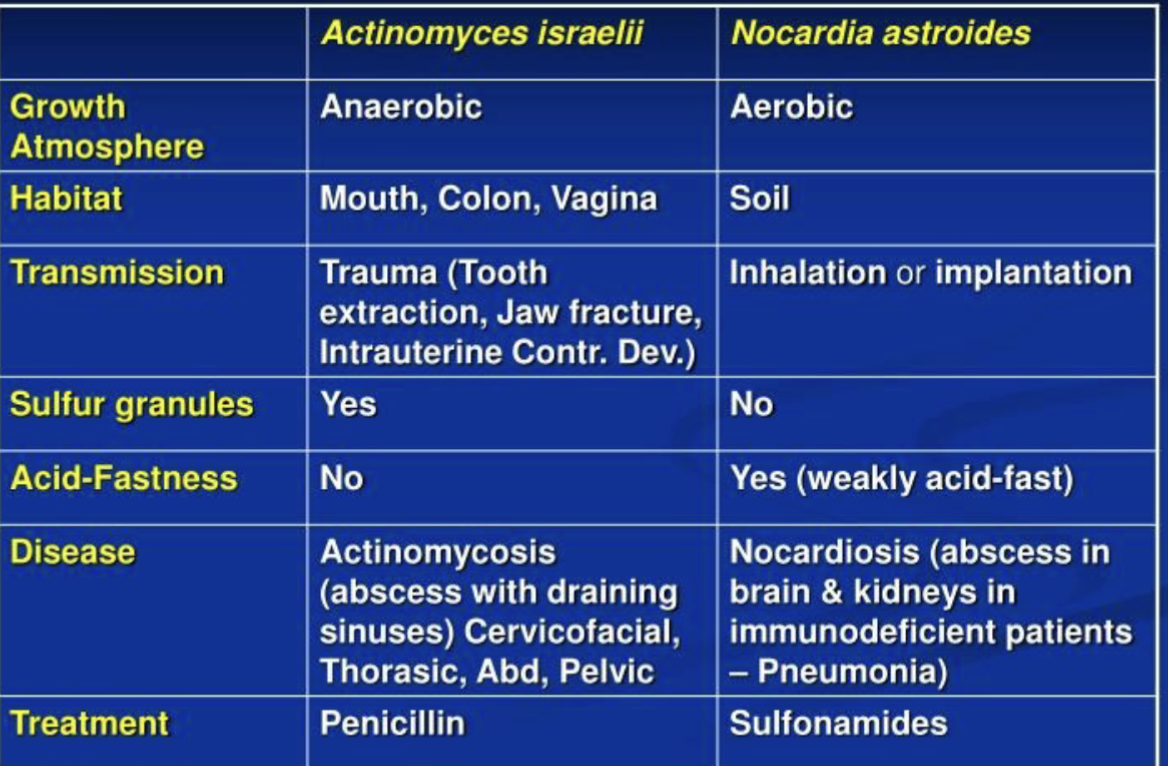
Differences between Actinomyces and Nocardia
Nocardia common manifestations:
cutaneous disease: mycetoma or actinomycetoma
pulmonary (similar to TB)
systemic
CNS disease
N. asteroides complex including N. facinica and N. nova cause?:
Most serious systemic and CNS diseases
N. brasiliensis causes?:
Cutaneous disease mostly in tropical countries and southeastern of USA
N. pseudobrasiliensis causes?:
Systemic infections, including the CNS
Nocardia diagnosis:
Grow in BAP, Sabouraud, brain heart infusion (BHI)
prolonged incubation may be required for their detection (up to 2 weeks)
grow on most nonselective media
specimens with mixed flora can over grow the nocardia colonies
Selective media that may increase yield:
–Thayer-Martin agar with antibiotics
– Buffered charcoal-yeast extract (BCYE) medium
Streptomyces general characteristics:
Second most commonly isolated Actinomycete
Catalase positive
Requires 2 to 3 weeks incubation for growth
Streptomyces microscopic morphology:
Gram positive branching bacilli
Right angle formations
Nonacid-fast
Form branching filaments of cells which become a network of strands called a mycelium
Streptomyces: colony morphology:
“spider” microcolonies
Glabrous, waxy, and heaped
Most are grayish-white
Observe aerial mycelium and conidia on slide cultures