07.10 BIO, HN U7P1 Proteins, Chemical Reactions, & Enzymes (ALL)
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
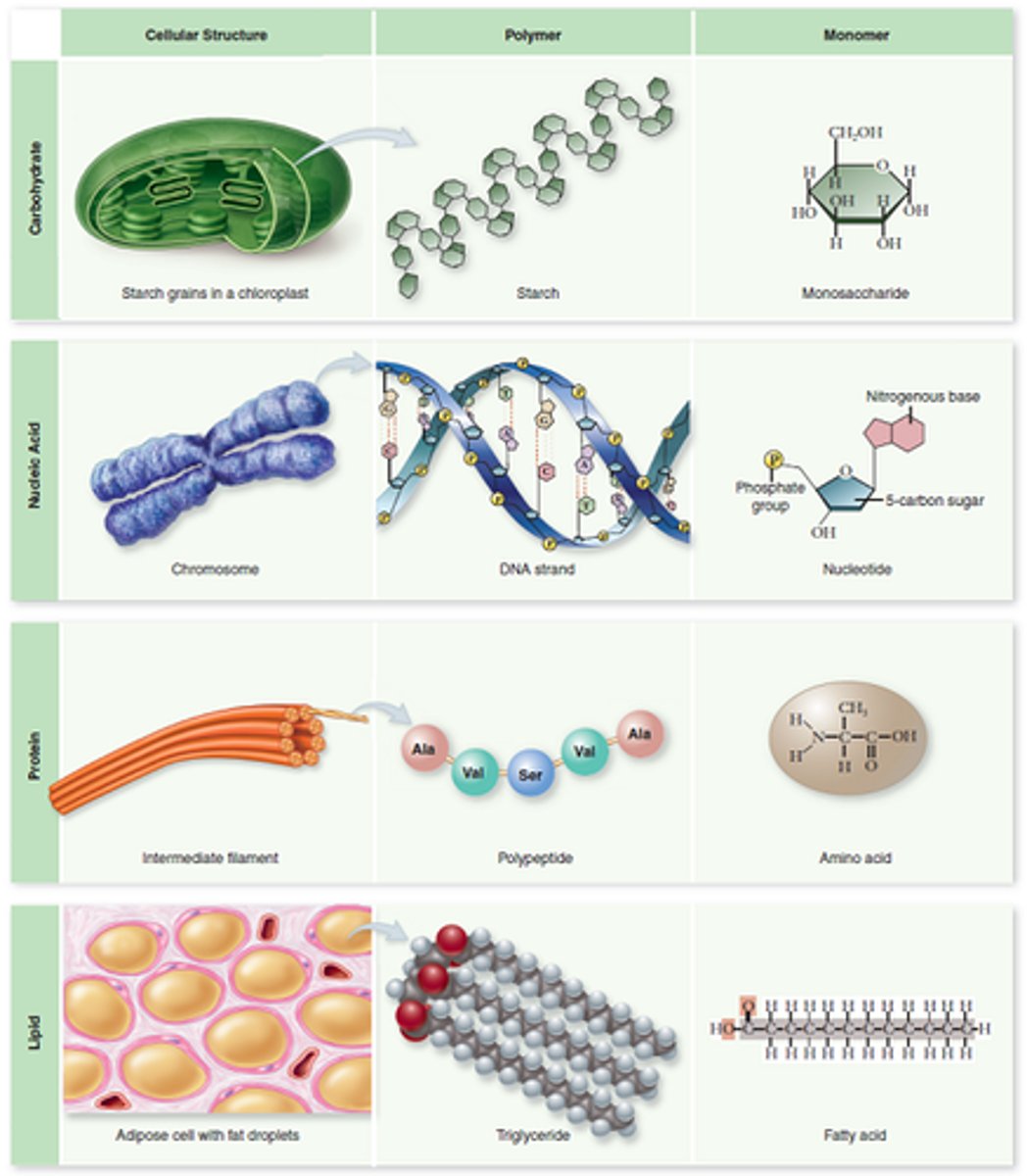
Macromolecules
A large molecule formed by the joining of smaller molecules called monomers. Organic macromolecules include carbohydrates, lipids, proteins, and nucleic acids.
Hydrolysis
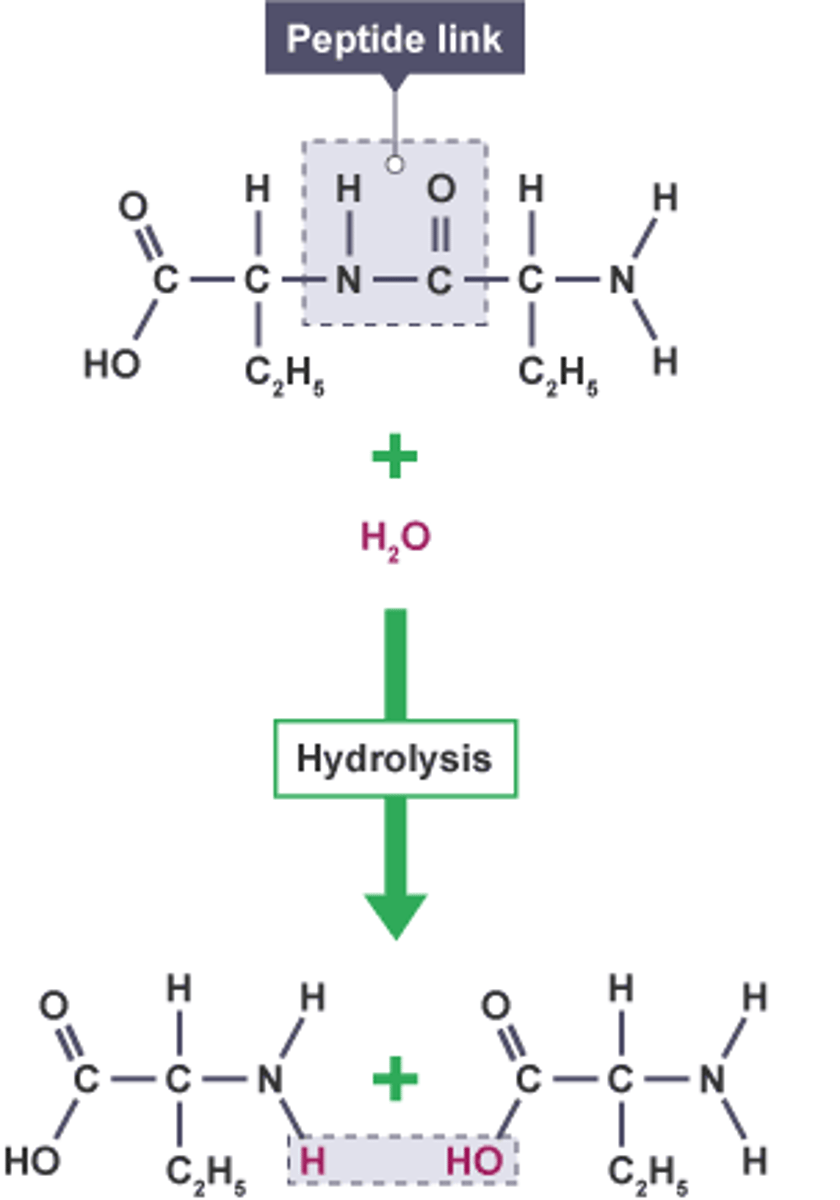
Process in which water is added and acts like a knife to break the bond between the monomers; one water is required to break a single bond
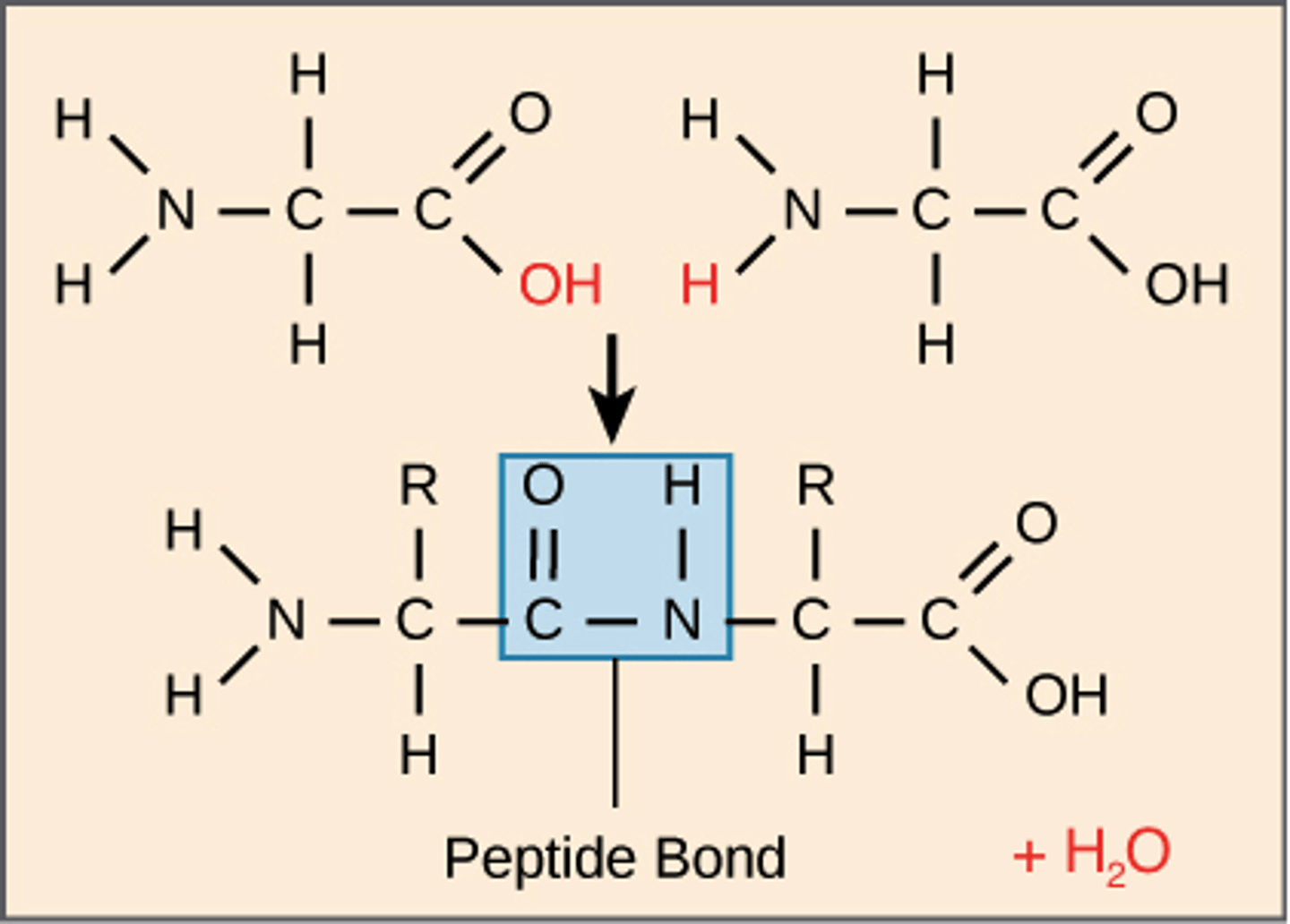
Dehydration synthesis
A chemical reaction in which two molecules covalently bond to each other with the removal of a water molecule; for each bond broken one water molecule must be removed
-pep
Root of word that typically indicates a protein
Amino acid
Monomers that make up protein. Consist of a central carbon with a hydrogen and one of twenty R-groups attached, along with an amino group (-NH₂) on one end, a carboxyl group (-COOH) on the other end.
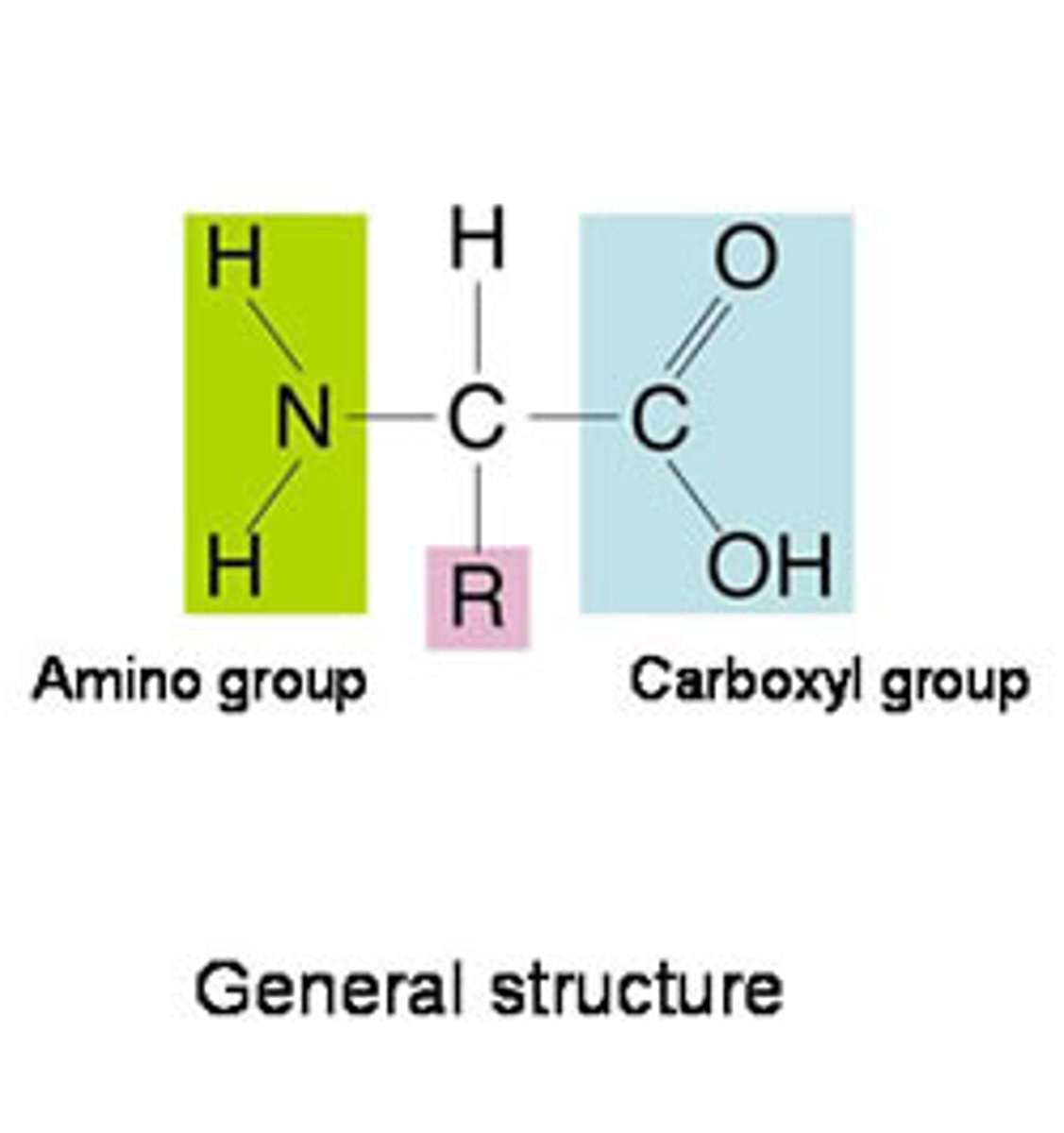
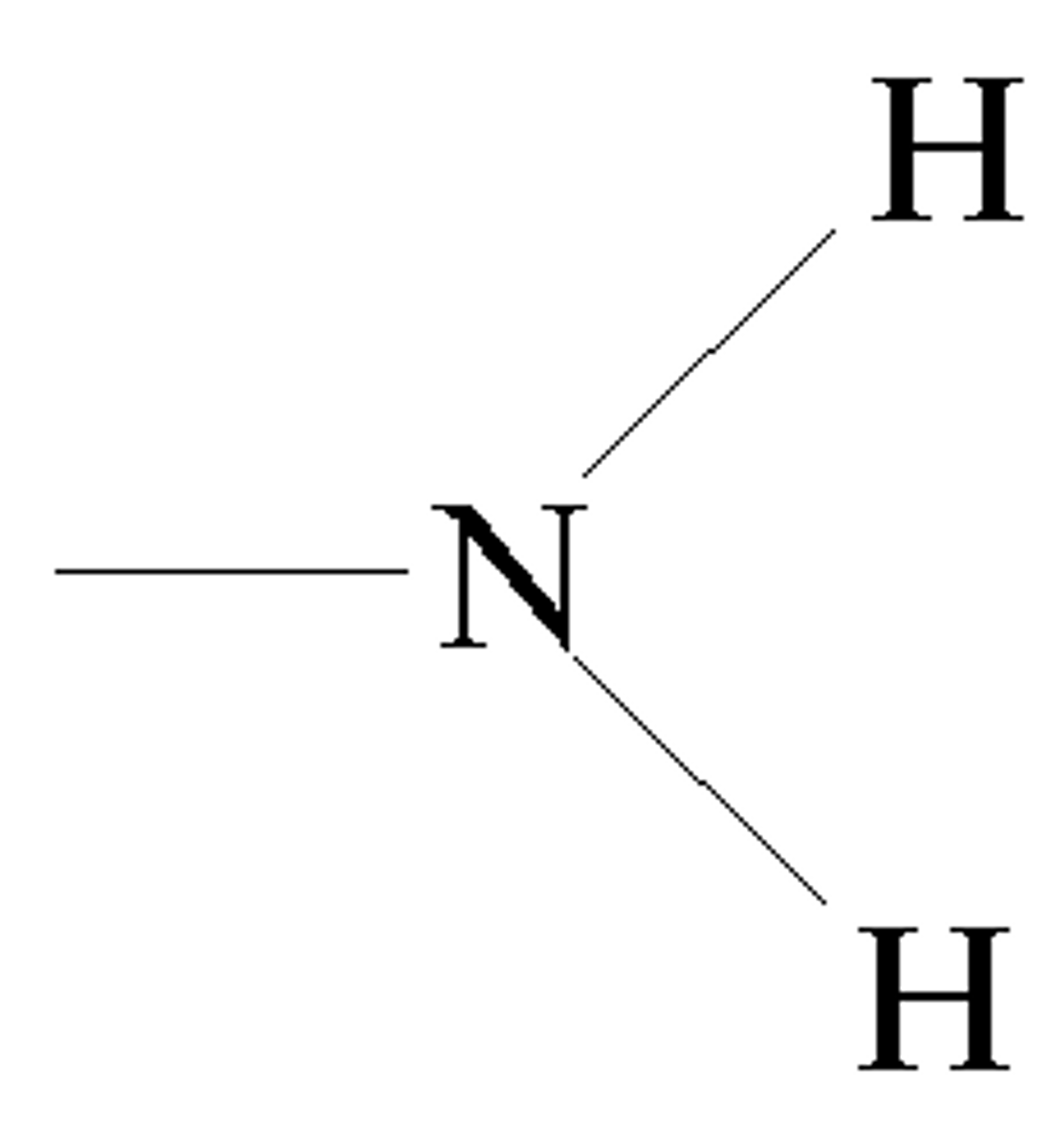
Amino group
A functional group present in organic molecules that consists of a nitrogen atom bonded to two hydrogen atoms
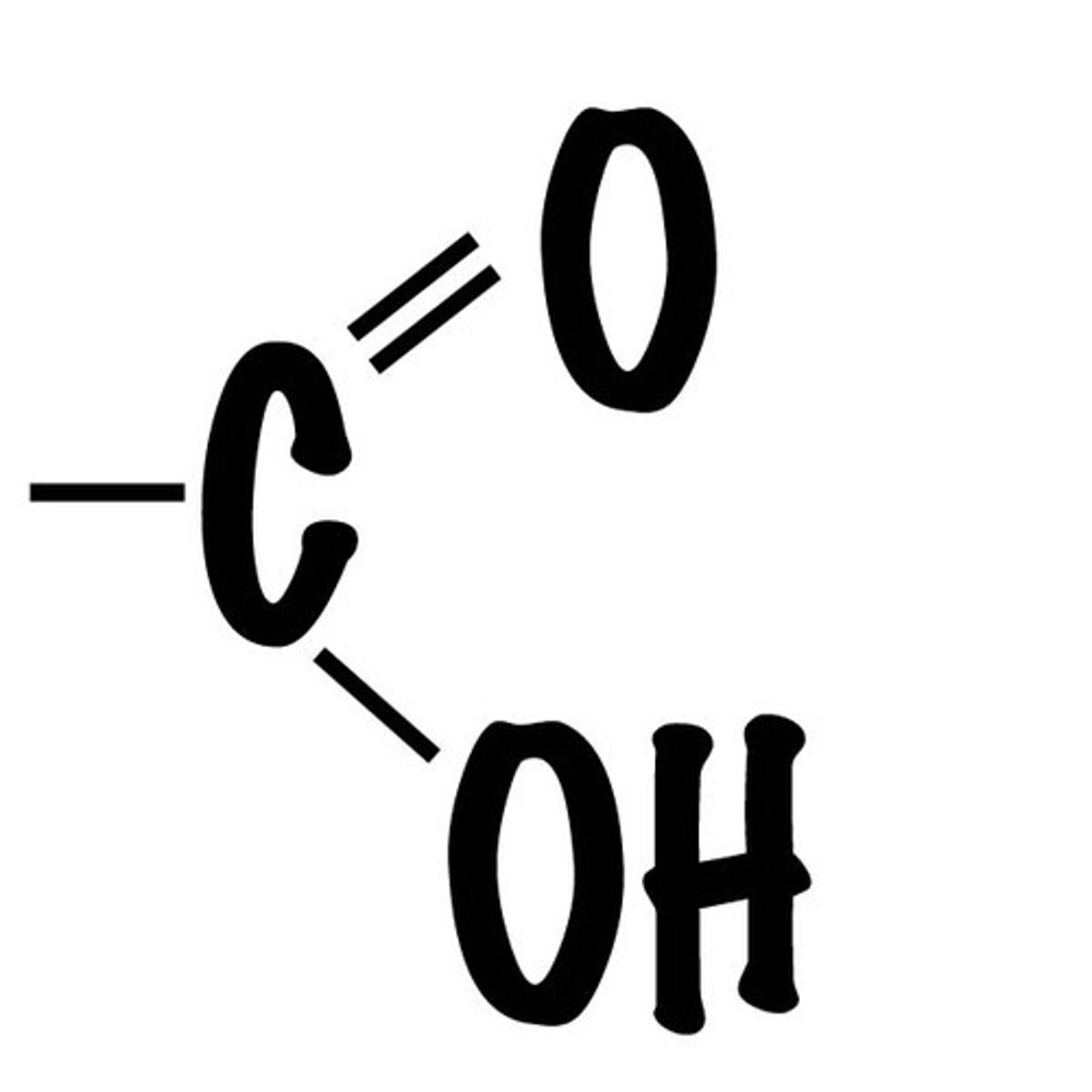
Carboxyl group
A functional group present in organic acids that consists of a single carbon atom double-bonded to an oxygen atom and also bonded to a hydroxyl group.
Peptide bond
The covalent bond between two amino acids, joining them into a peptide or protein.
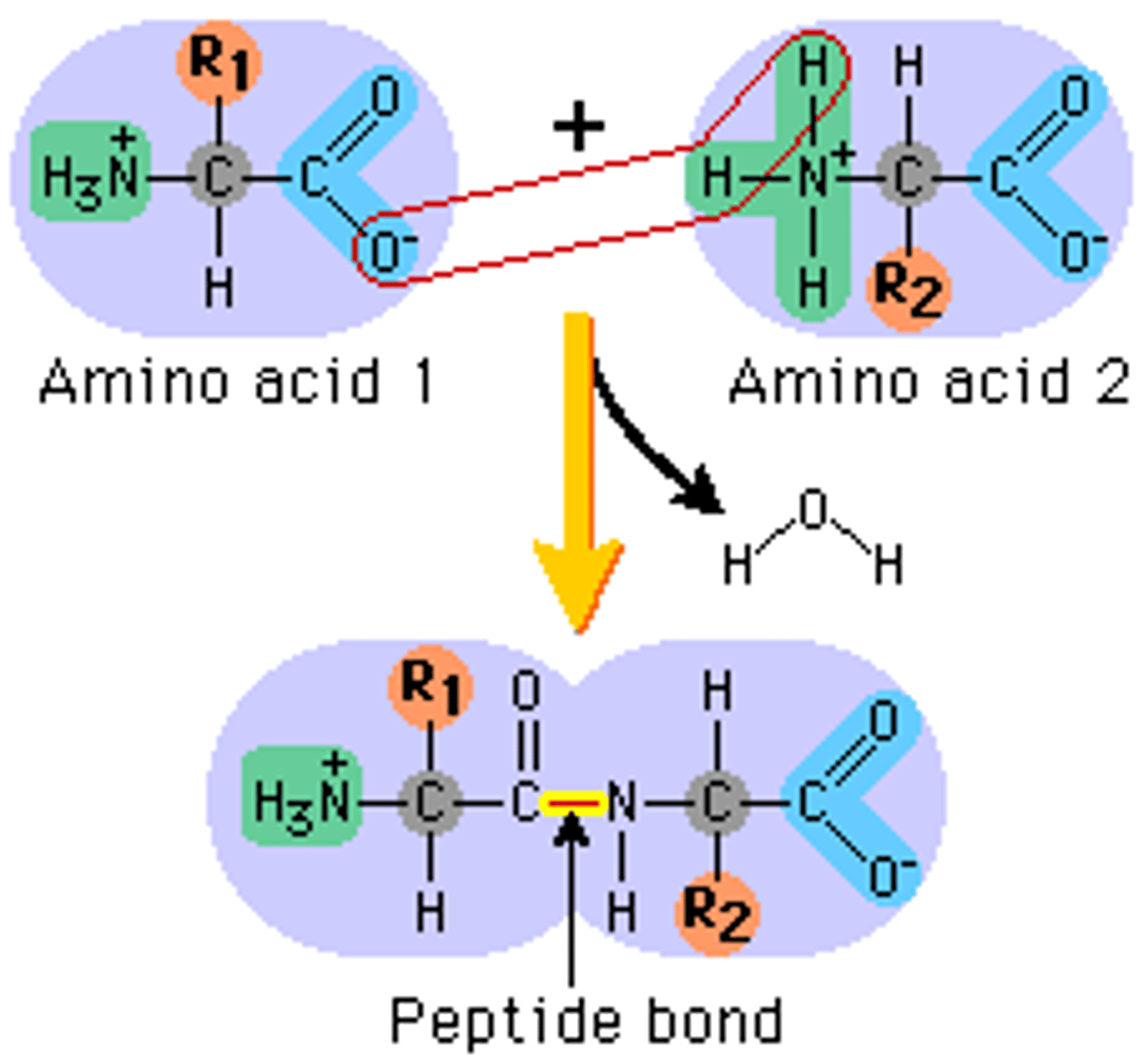
Dipeptide
Protein made of two amino acids bonded together by a peptide bond.
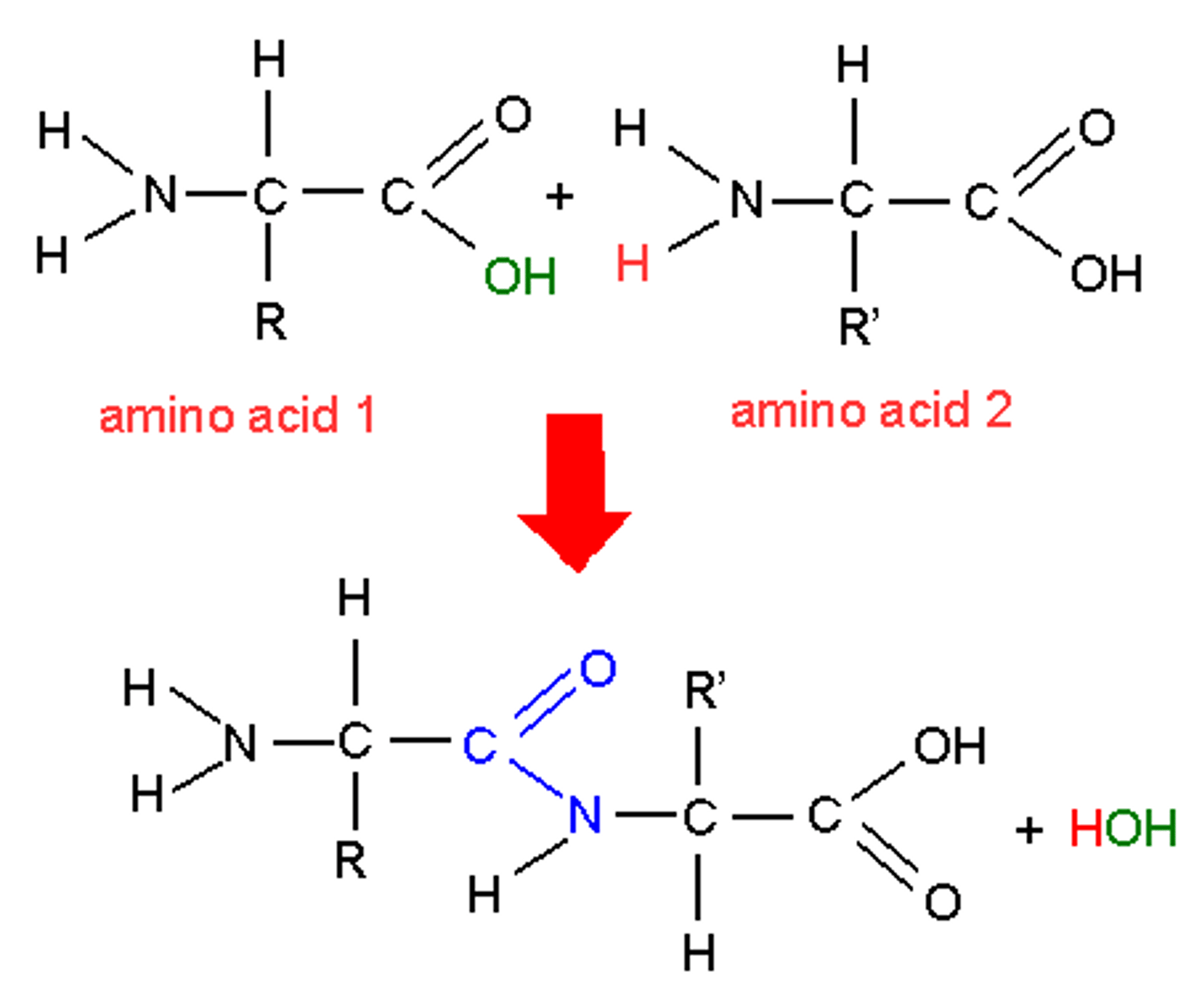
Polypeptide
Protein made of many amino acids bonded together by peptide bonds.
Protein (Description)
A macromolecule found in living things that makes up the structure of skin, hair and nails, helps regulate chemical reactions, transports materials, involved in muscle contraction and can help fight infection.
Proteins (Elements)
A macromolecule that is made up carbon, hydrogen, oxygen, nitrogen and sometimes sulfur
Proteins (Monomers)
A macromolecule that is made of amino acids
Proteins (Examples)
Examples include actin, collagen, hemoglobin, keratin, myosin, DNA polymerase, helicase, ATP synthase
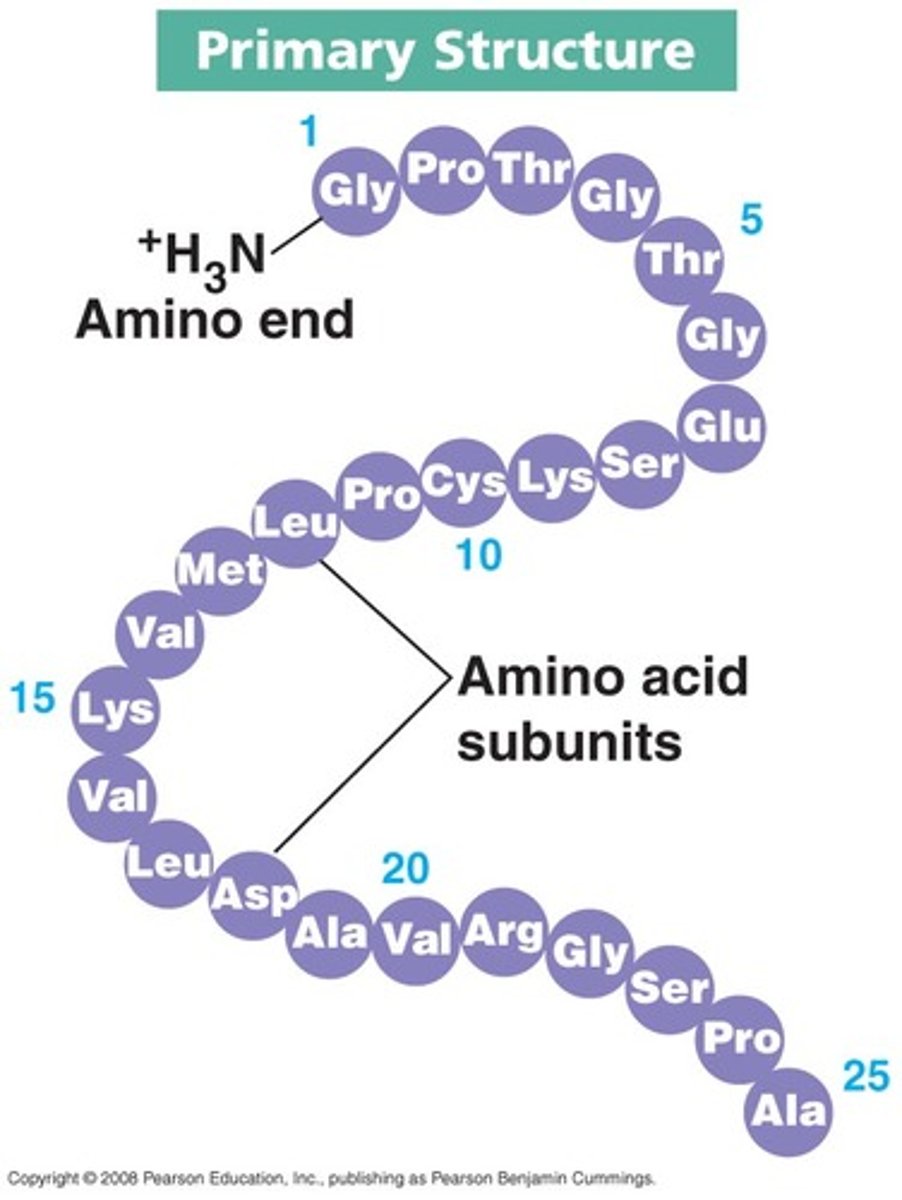
Protein (Primary Structure)
The unique sequence of amino acids that make up a polypeptide
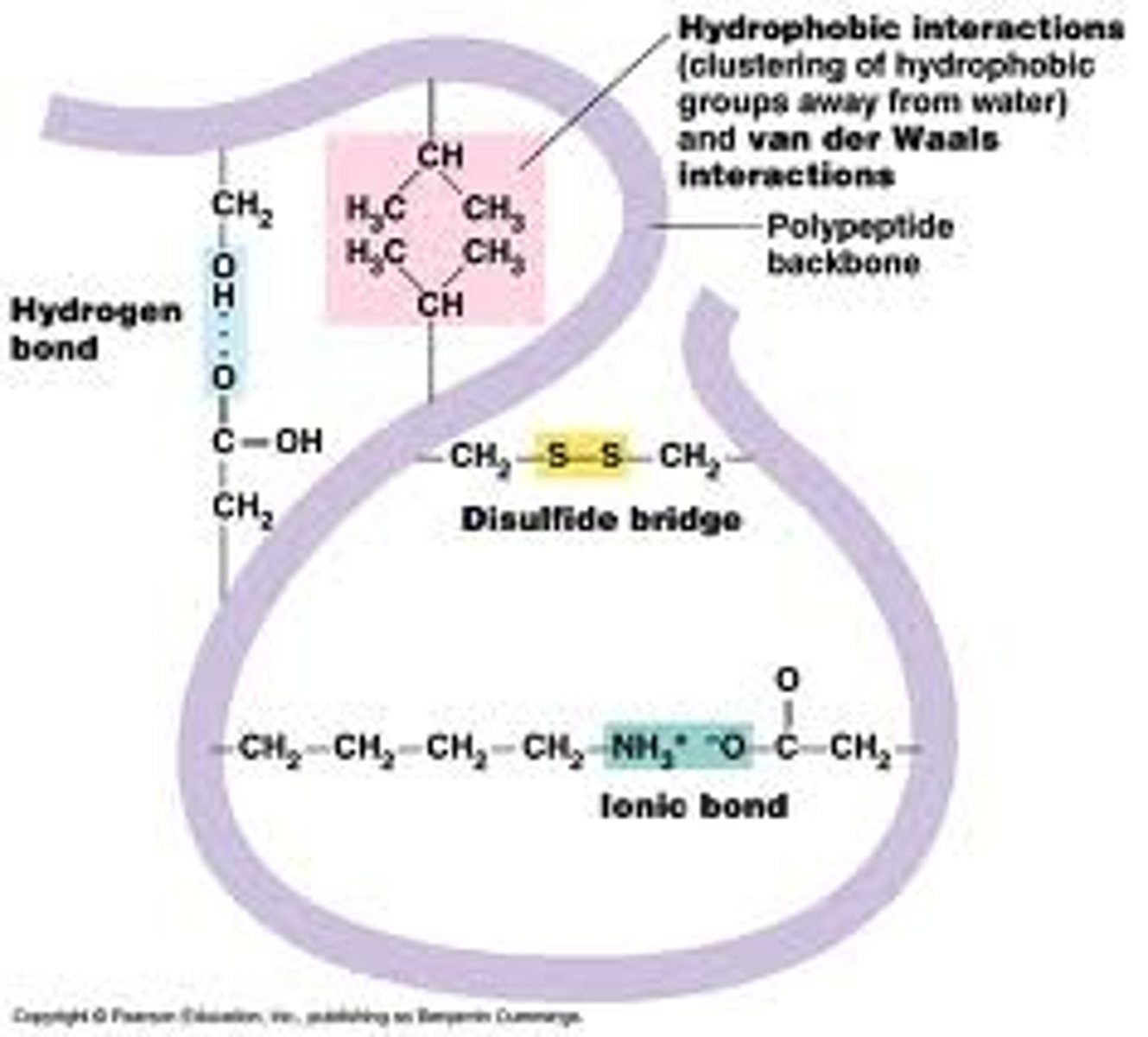
Protein (Tertiary Structure)
The bending and folding of a protein secondary structure due to the interactions of the R-groups on each amino acid that creates the 3-D shape of a polpypeptide; includes hydrophobic interactions, hydrogen bonds, ionic bonds, disulfide bridges
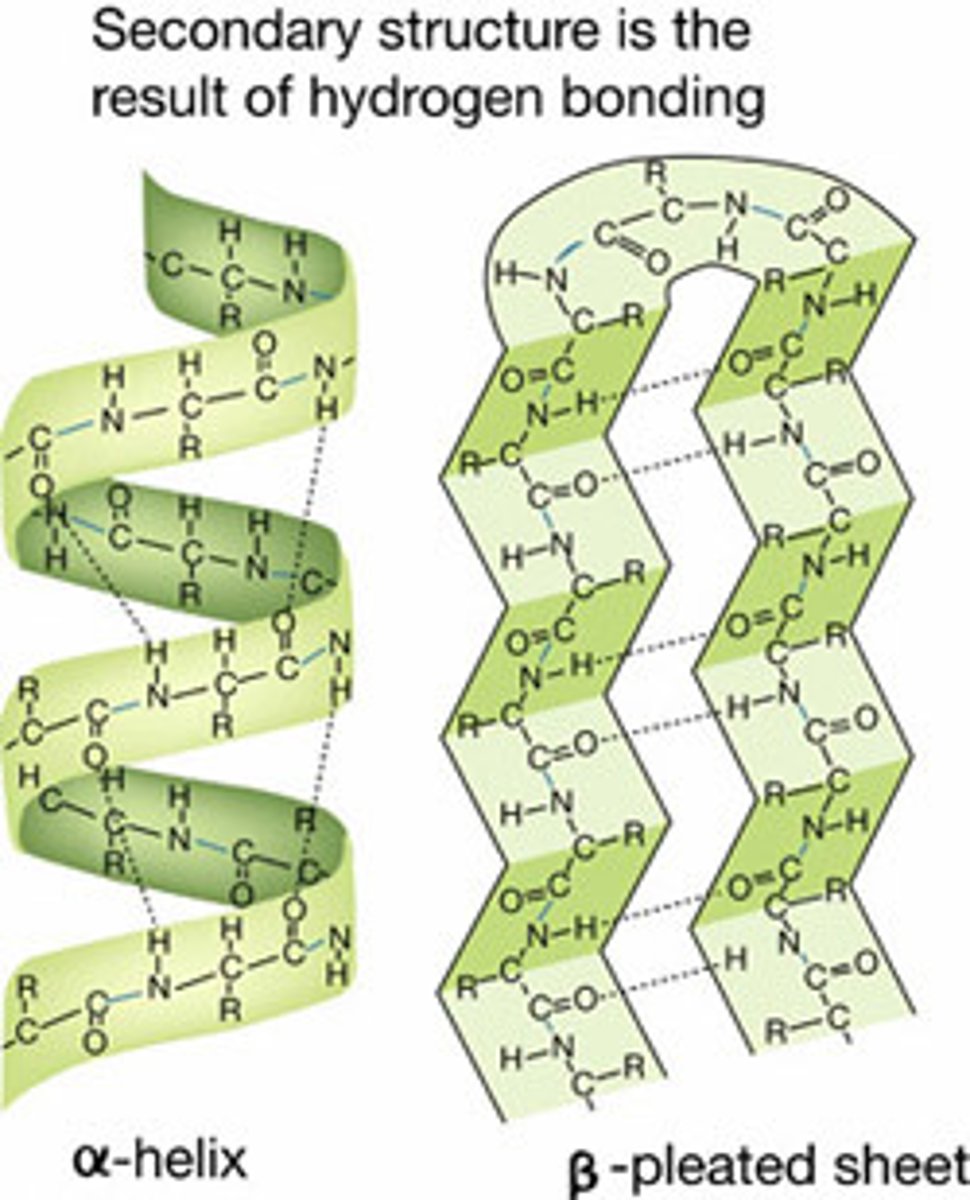
Protein (Secondary Structure)
Hydrogen bonding between carboxyl and amino of amino acids within a polypeptide that create alpha helices and beta pleated sheets
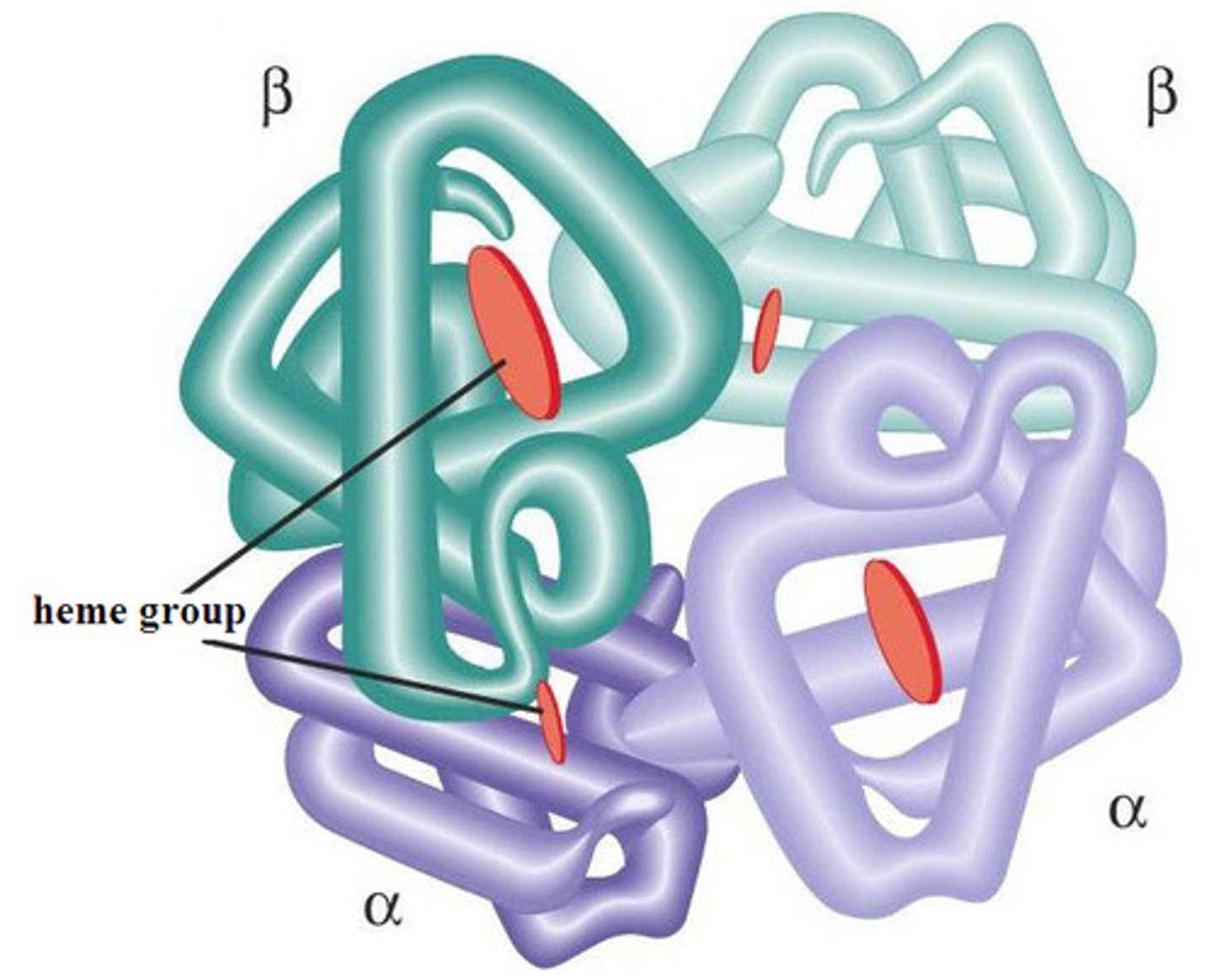
Protein (Quaternary Structure)
Structure that occurs when 2 or more polypeptides are bonded together to make a protein; does not occur in all proteins
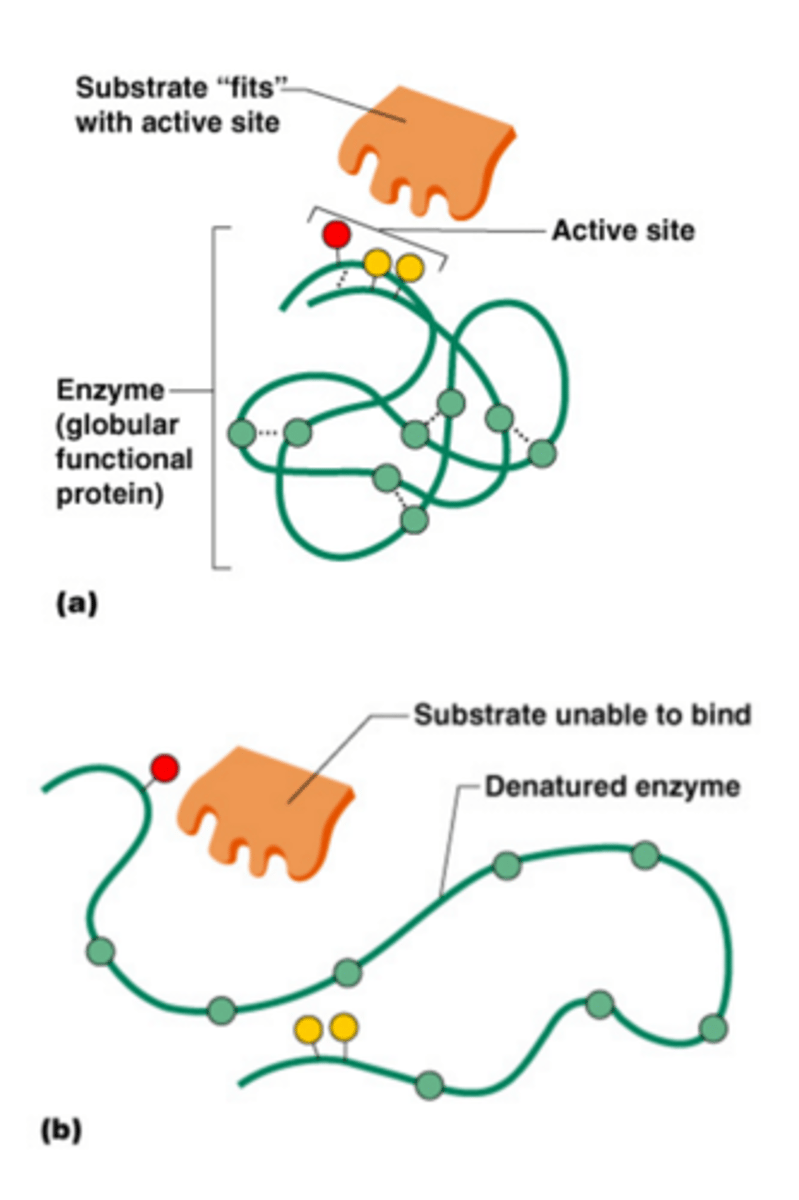
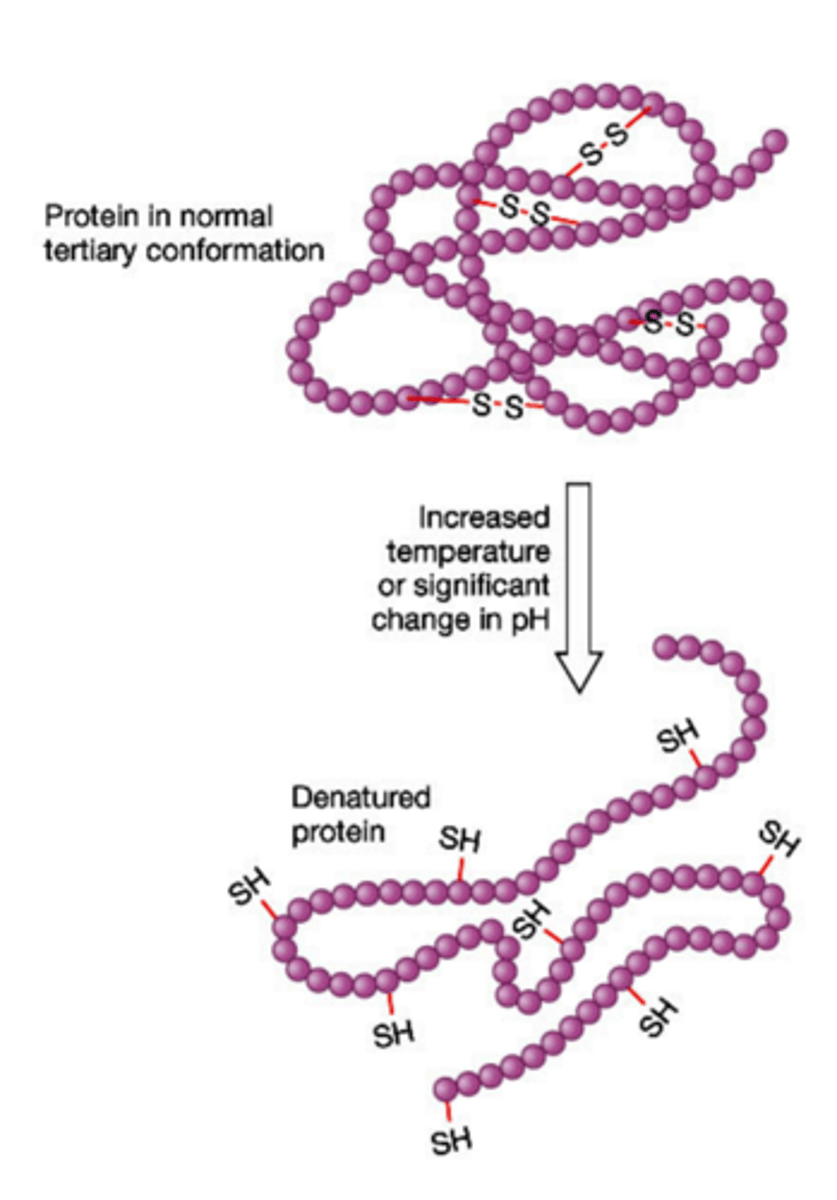
Denature
A change in the shape of a protein (such as an enzyme) that stops the protein from functioning. Can be caused by changes in conditions like temperature or pH
Enzyme
A protein that acts as a catalyst and speeds up chemical reactions in a living thing by lowering the energy needed to get the reaction going.
-ase
Ending on word typically indicates an enzyme
Collagen
A fibrous protein found in the skin and connective tissue that provides strength and support

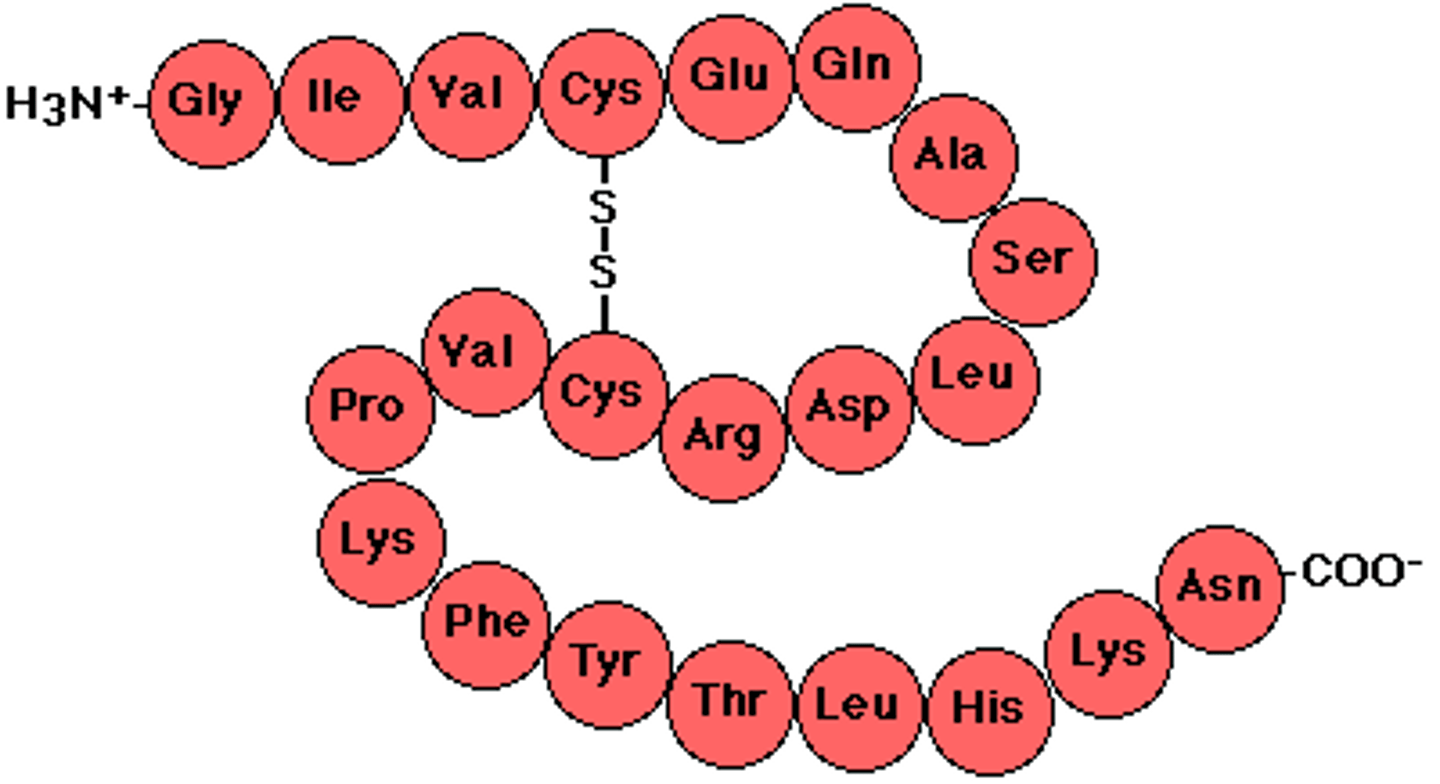
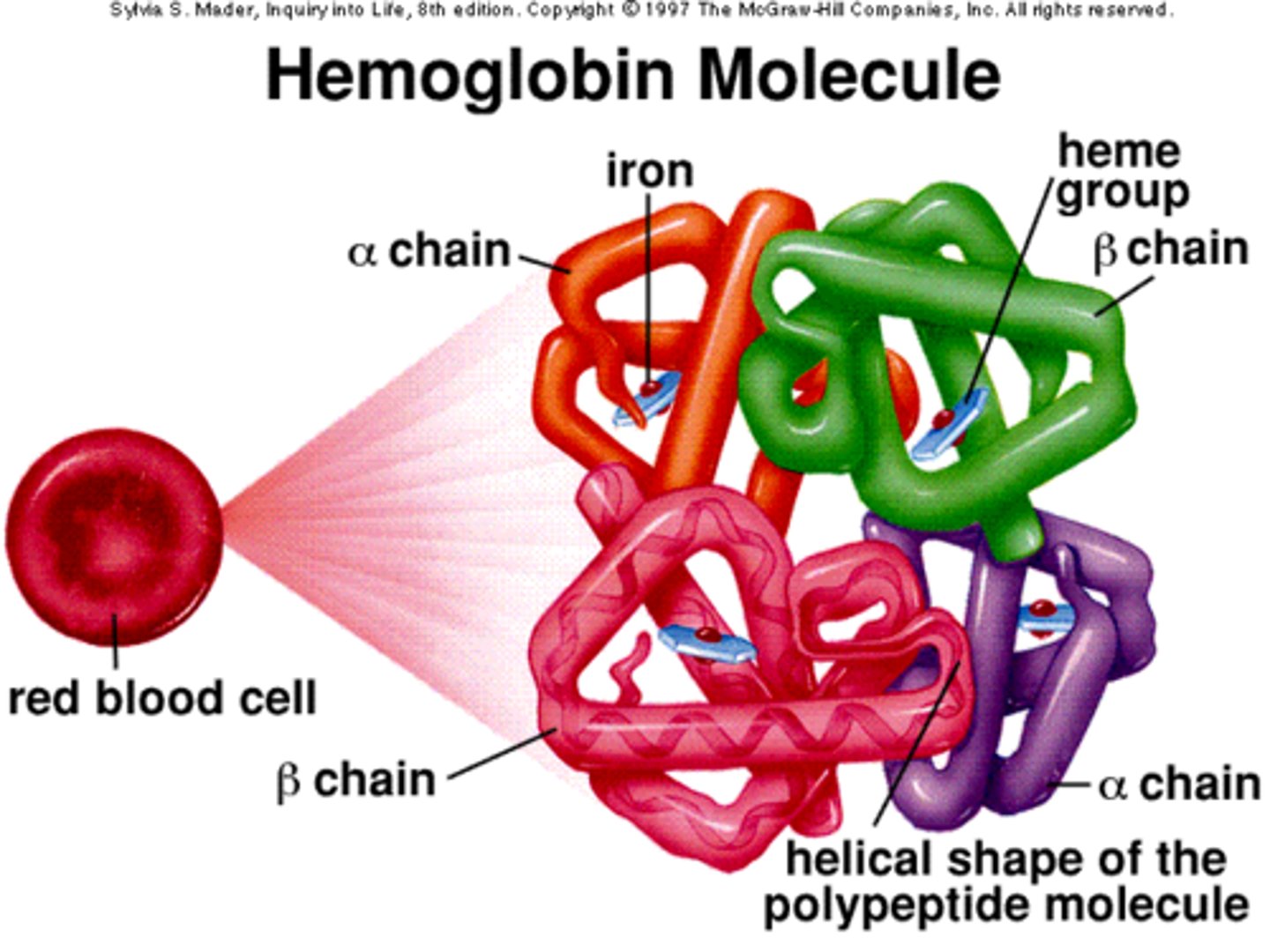
Hemoglobin
A four-subunit protein found in red blood cells that binds oxygen. Each subunit contains a heme group, a large multi-ring molecule with an iron atom at its center. One hemoglobin molecule can bind four oxygen molecules
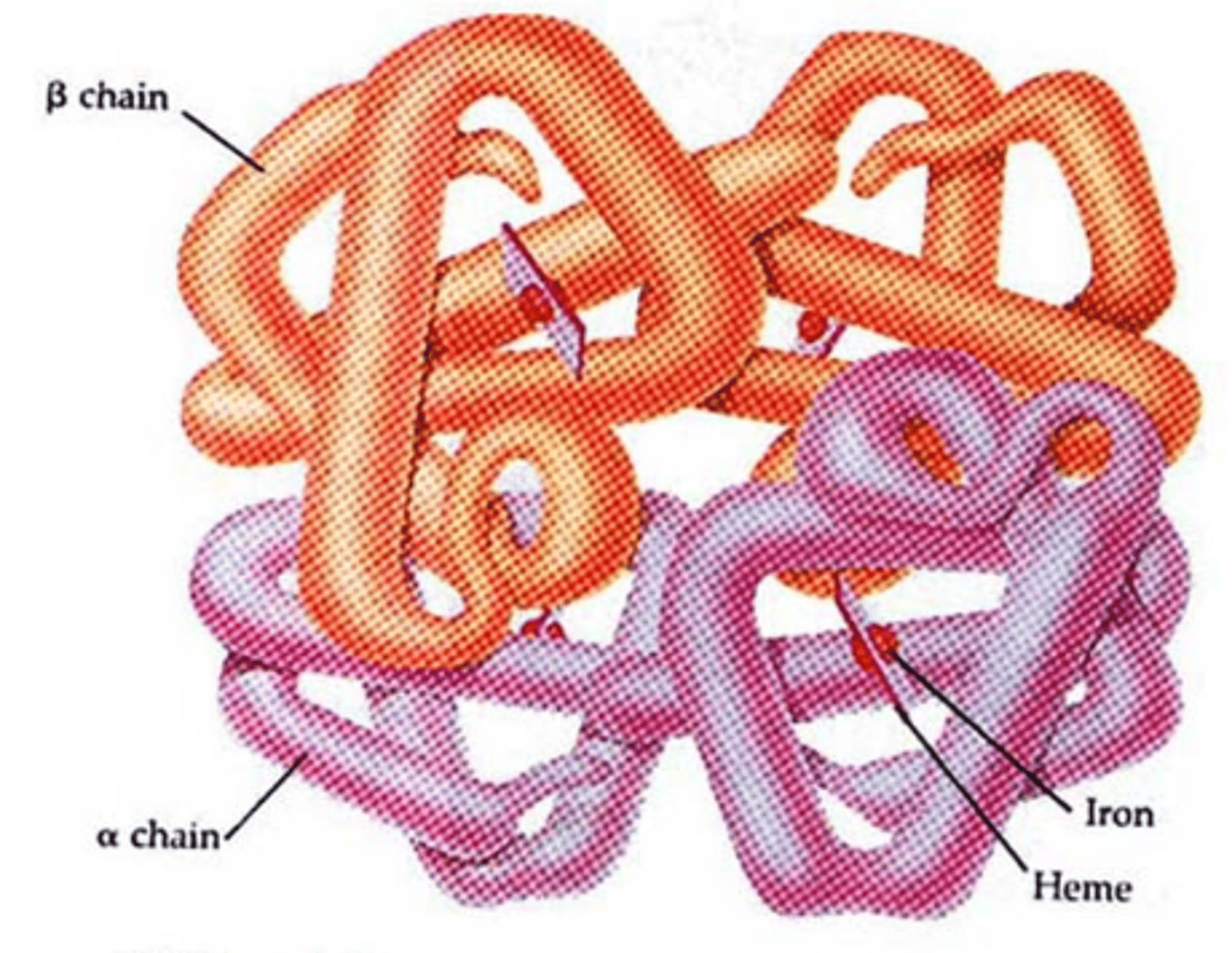
Keratin
A fibrous protein that is the principal component of hair, skin, and nails
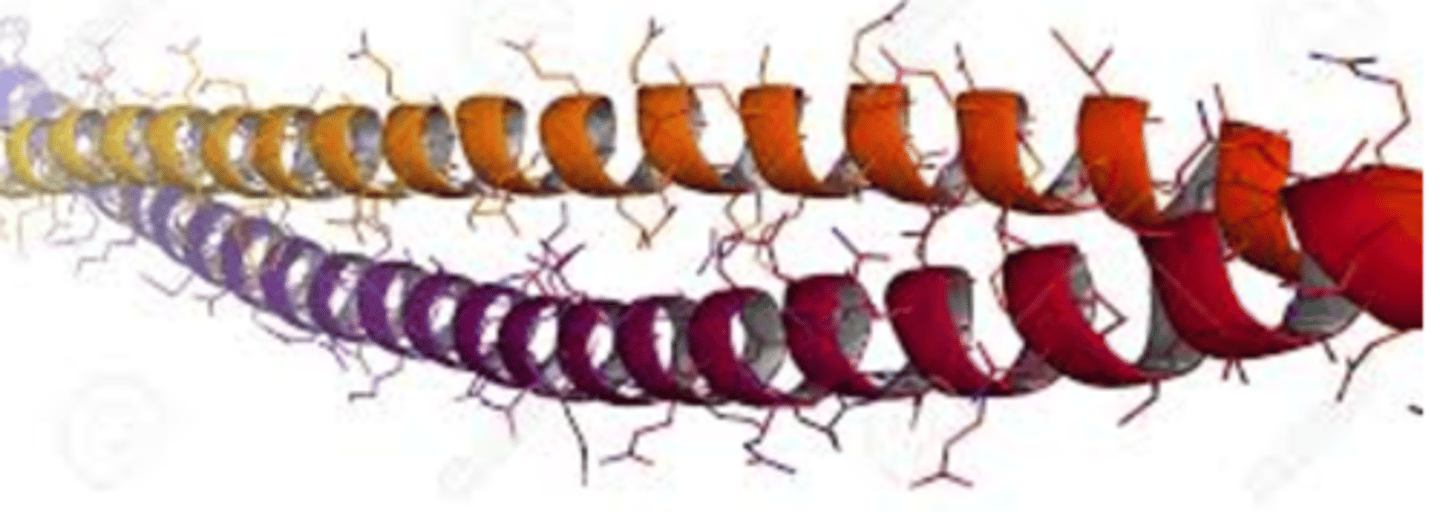
Actin
A globular protein that links into chains, two of which twist helically about each other, forming microfilaments in muscle and other contractile elements in cells
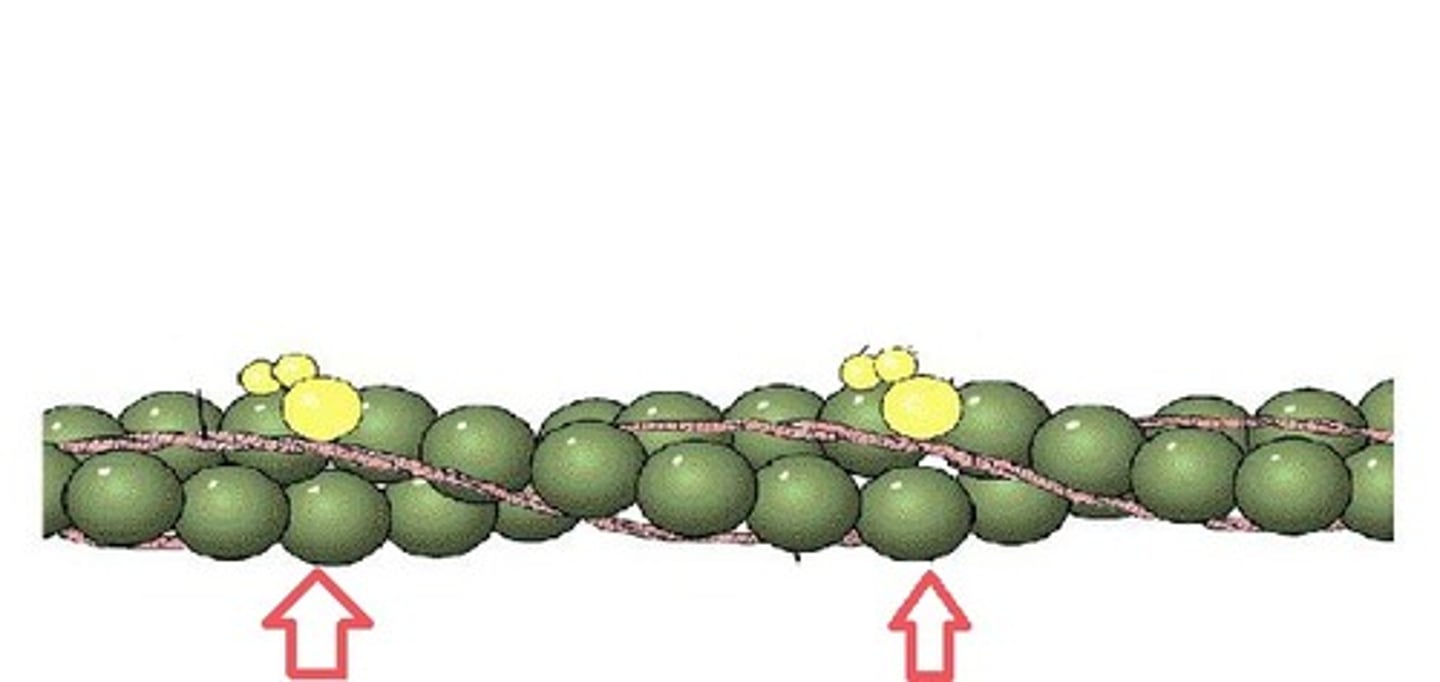
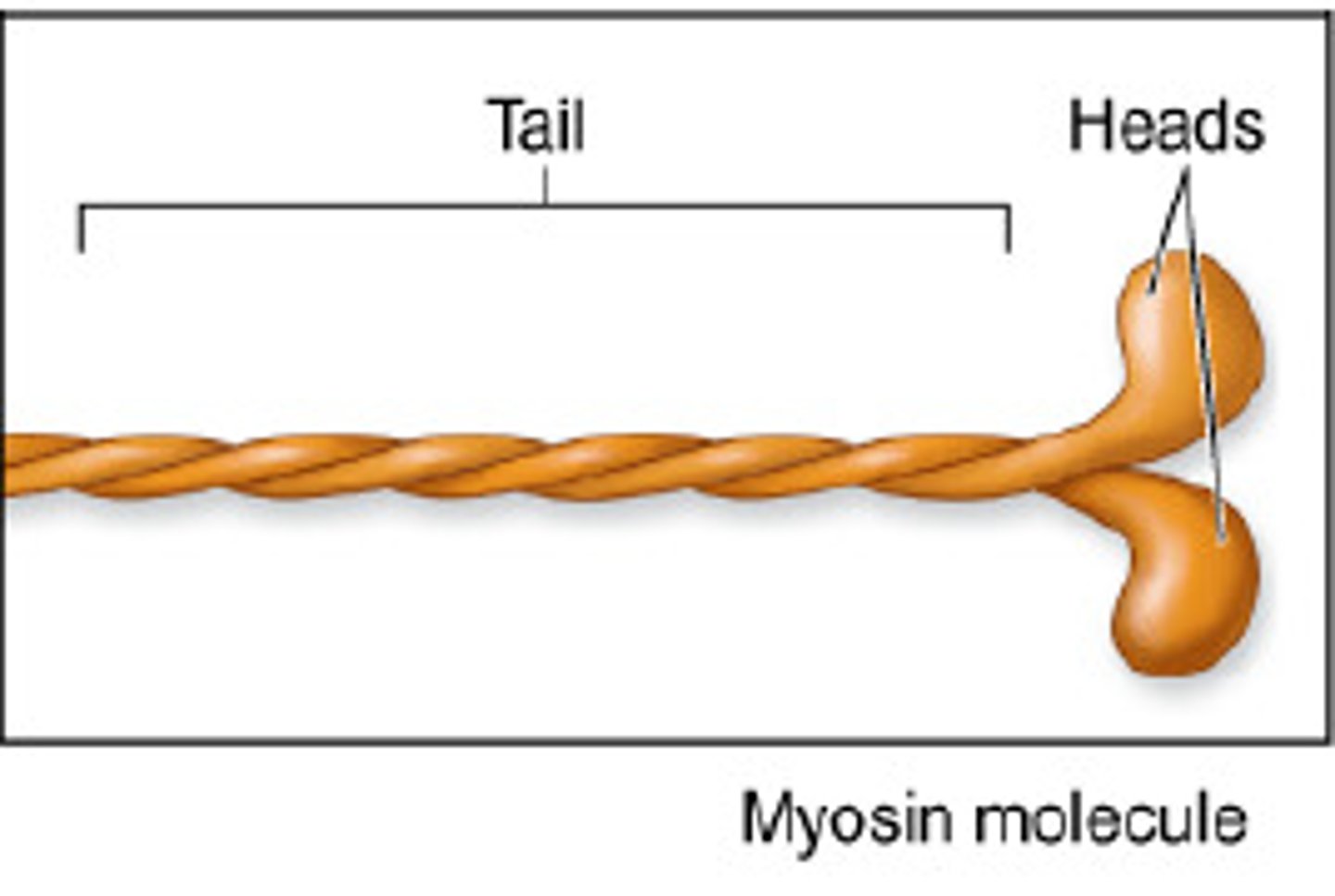
Myosin
A protein present in muscle fibers that aids in contraction and makes up the majority of muscle fiber
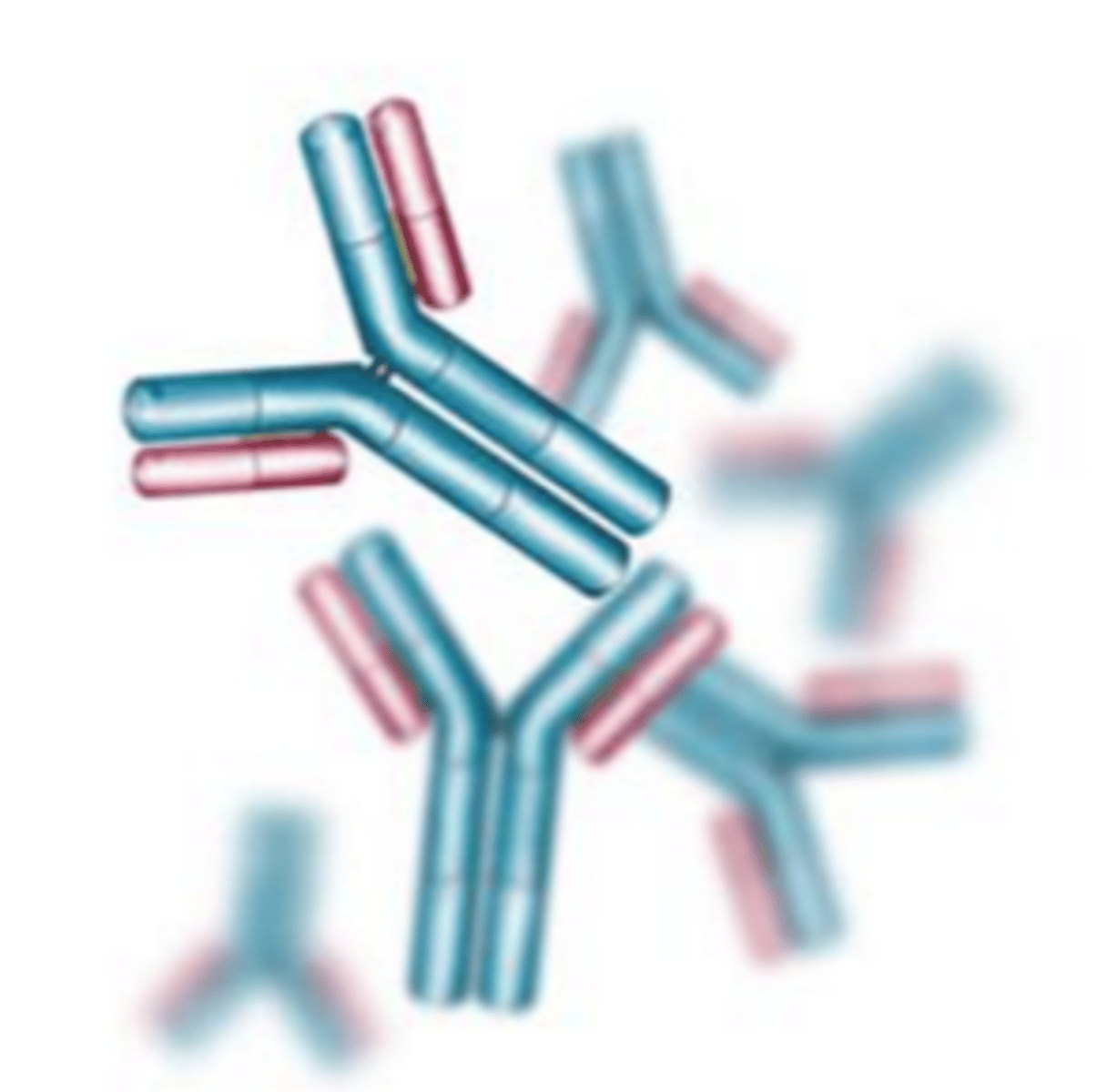
Antibodies
Specialized proteins that aid in destroying infectious agents
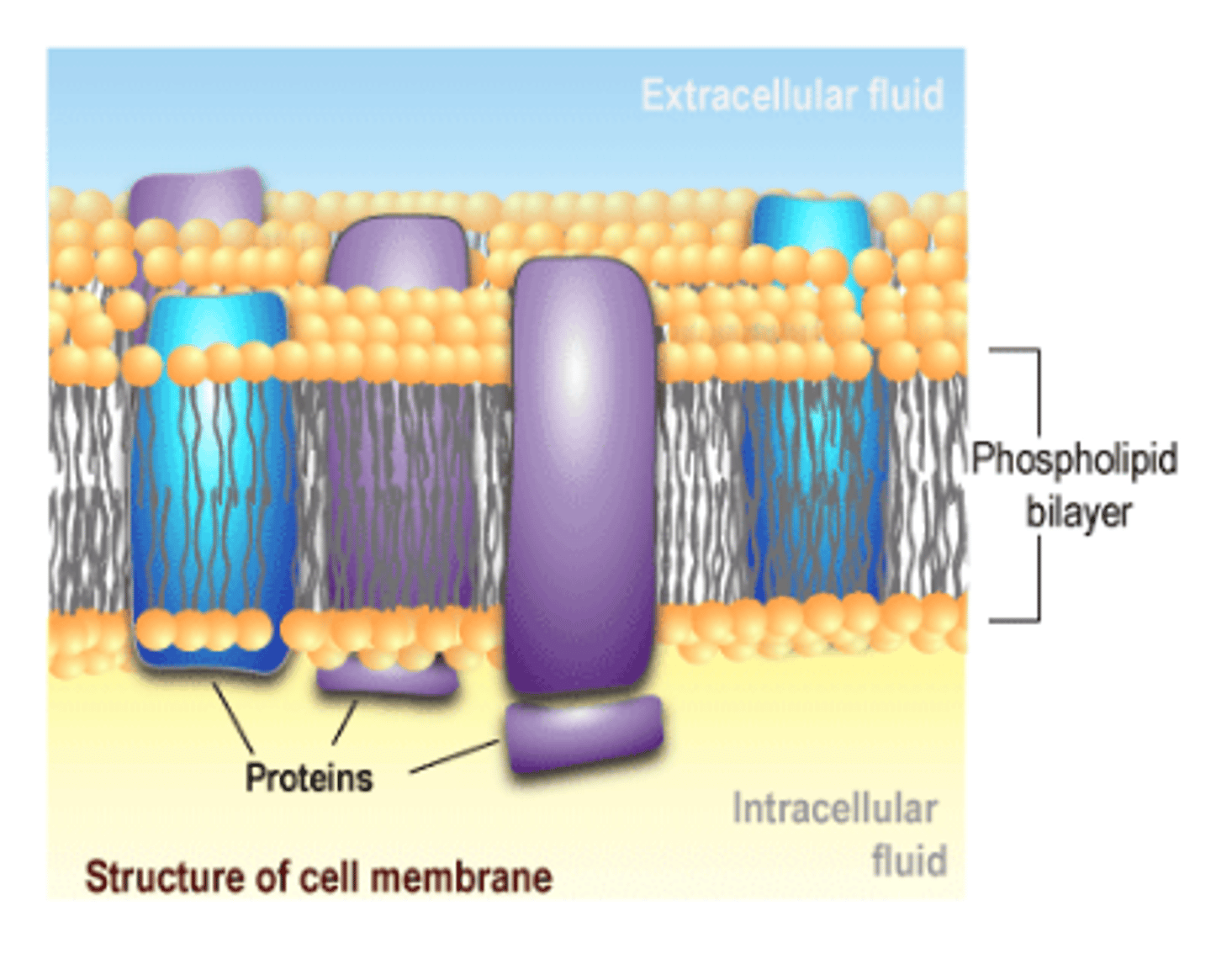
Membrane proteins
Proteins found within the cell membrane that transport materials, act as enzymes, serve as receptors and aid in cell-to-cell recognition
Chemical reaction
A process during which chemical bonds between atoms are broken and new ones are formed; producing one or more different substances
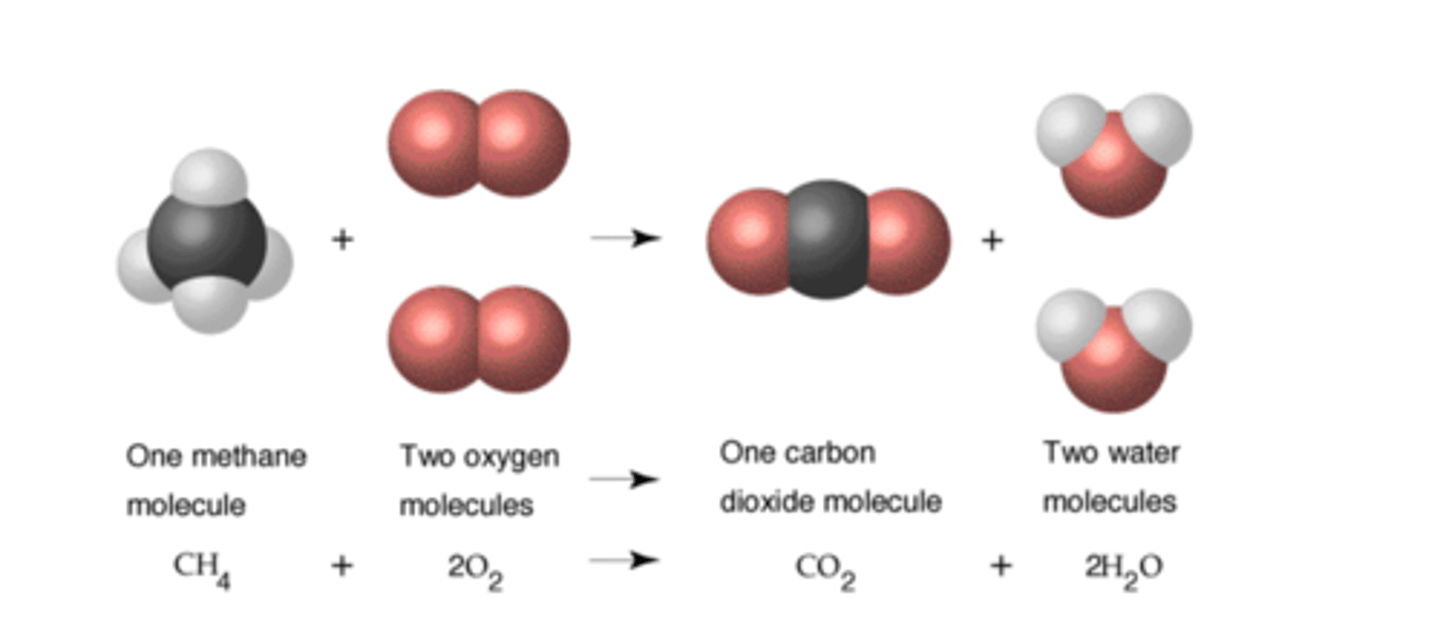
Reactants
Element or compound that enters into a chemical reaction that are found on the left side of the arrow in a chemical reaction.
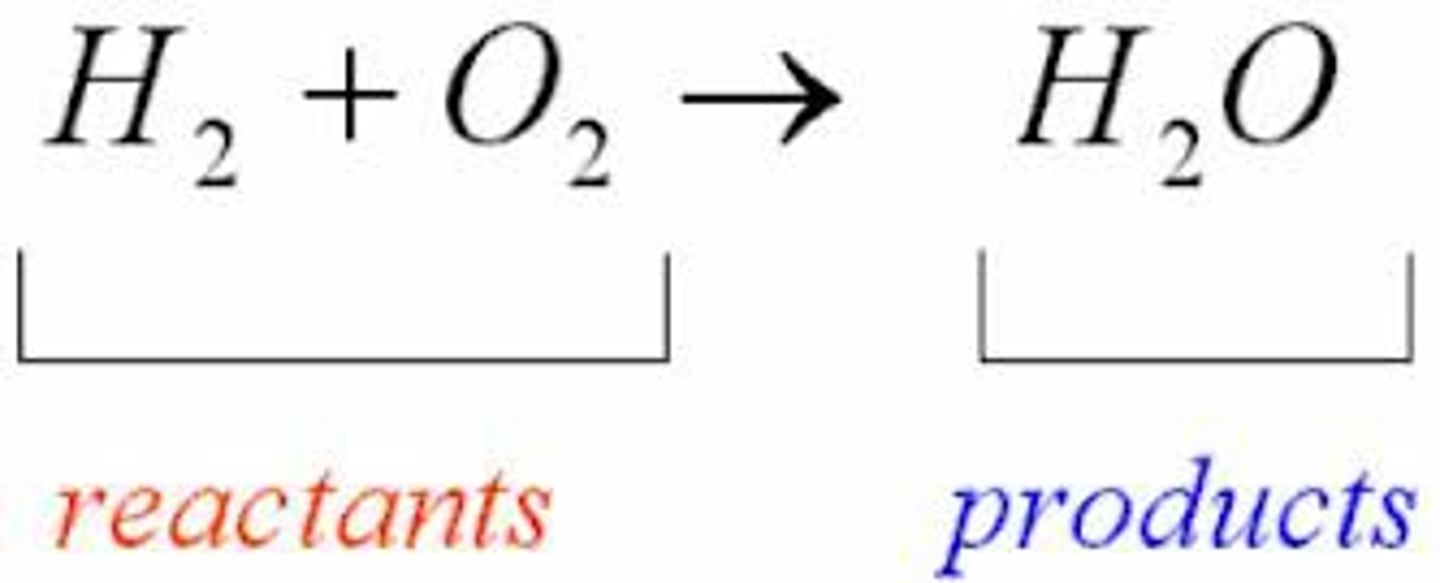
Products
Element or compound that are produced as a result of a chemical reaction that are found on the right side of the arrow in a chemical reaction
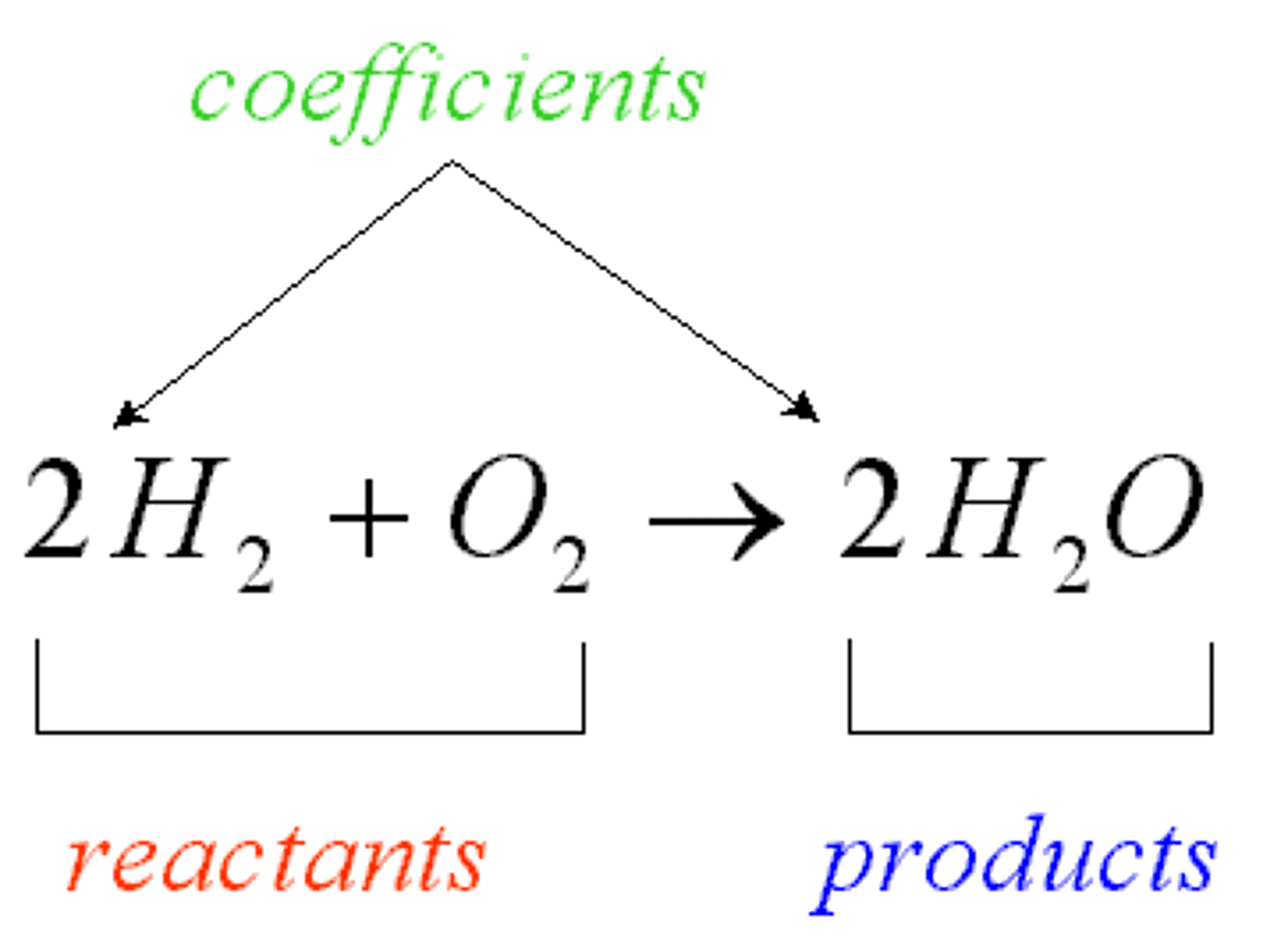
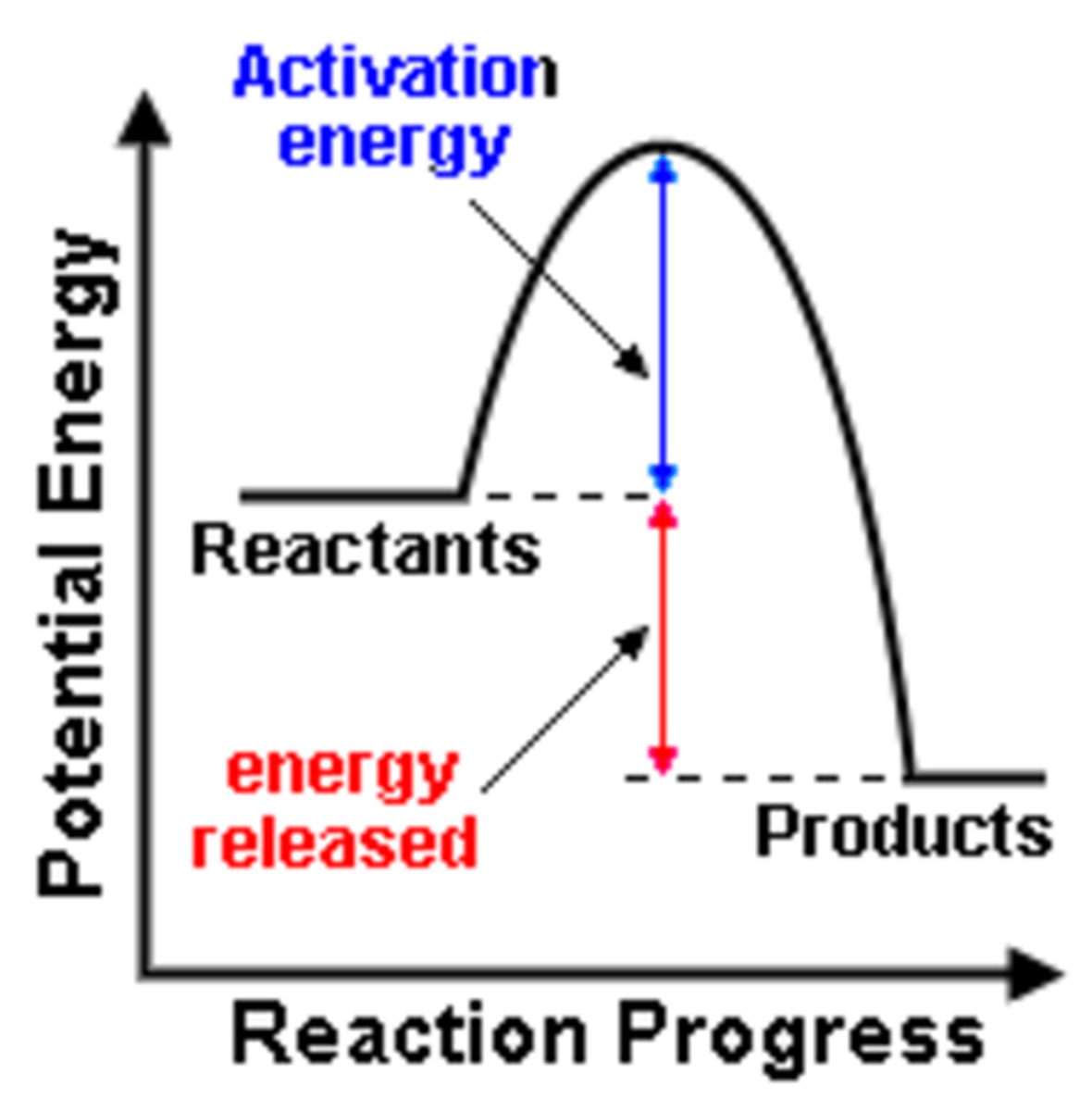
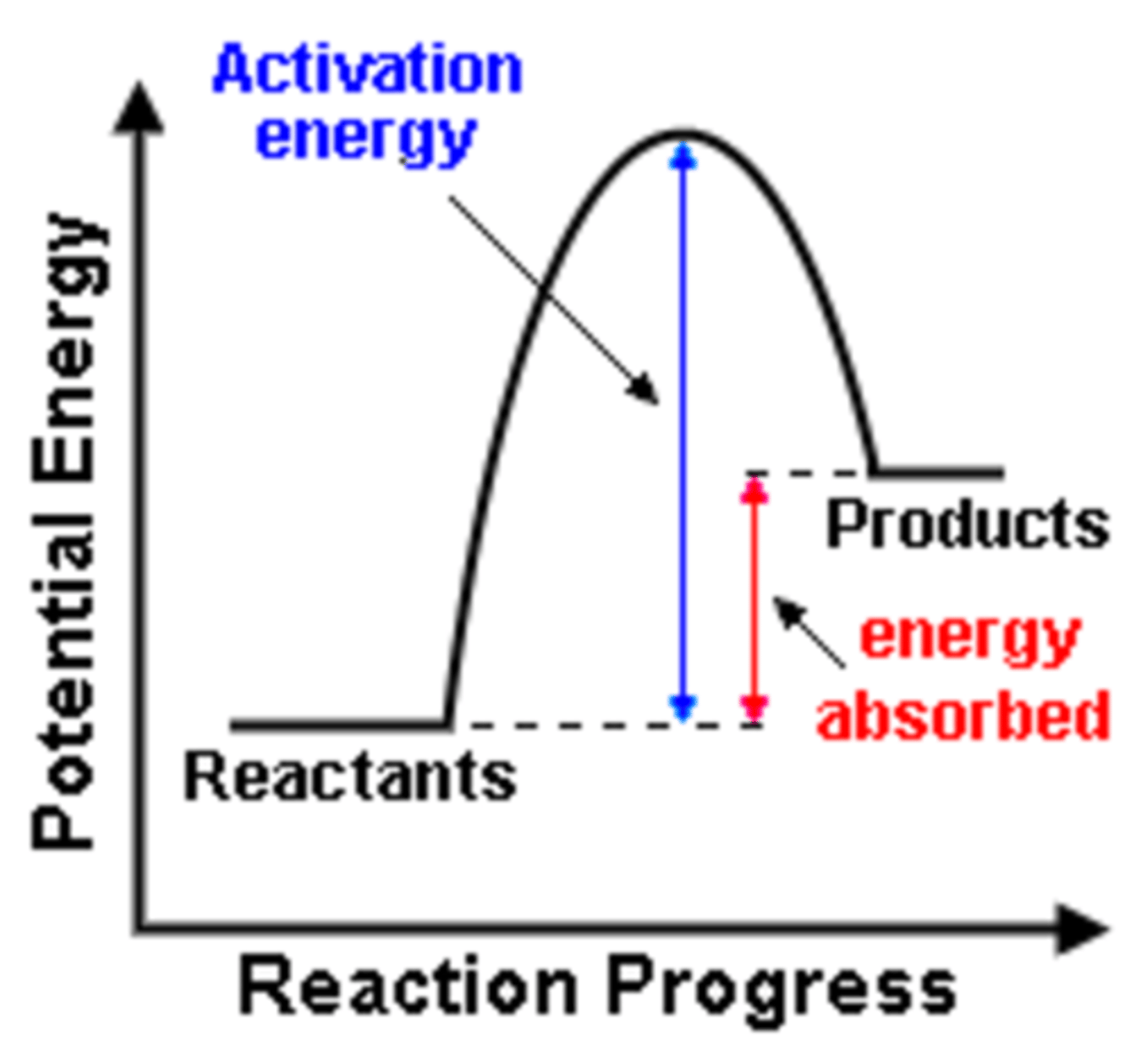
Activation energy
The minimum amount of energy needed to start a chemical reaction
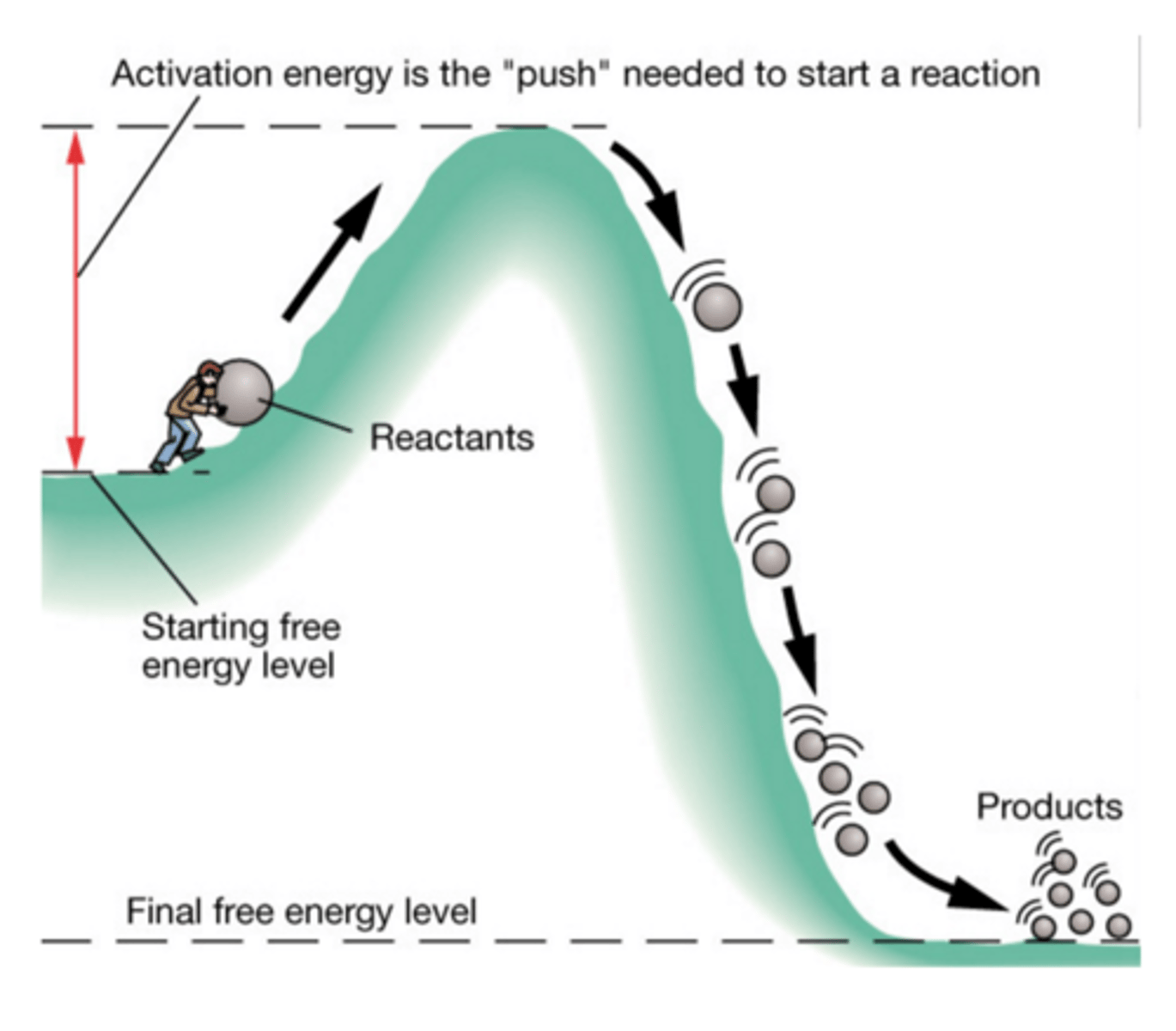
Exergonic or Exothermic reaction
A chemical reaction in which energy is released; often occurs spontaneously
Endergonic or Endothermic reaction
A chemical reaction in which energy is absorbed or stored; will not occur without a source of energy
Catalyst
A substance that speeds up the rate of a chemical reaction by lowering the energy required for the reaction to occur
Reactants
Element or compound that enters into a chemical reaction that are found on the left side of the arrow in a chemical reaction.
Products
Element or compound that are produced as a result of a chemical reaction that are found on the right side of the arrow in a chemical reaction
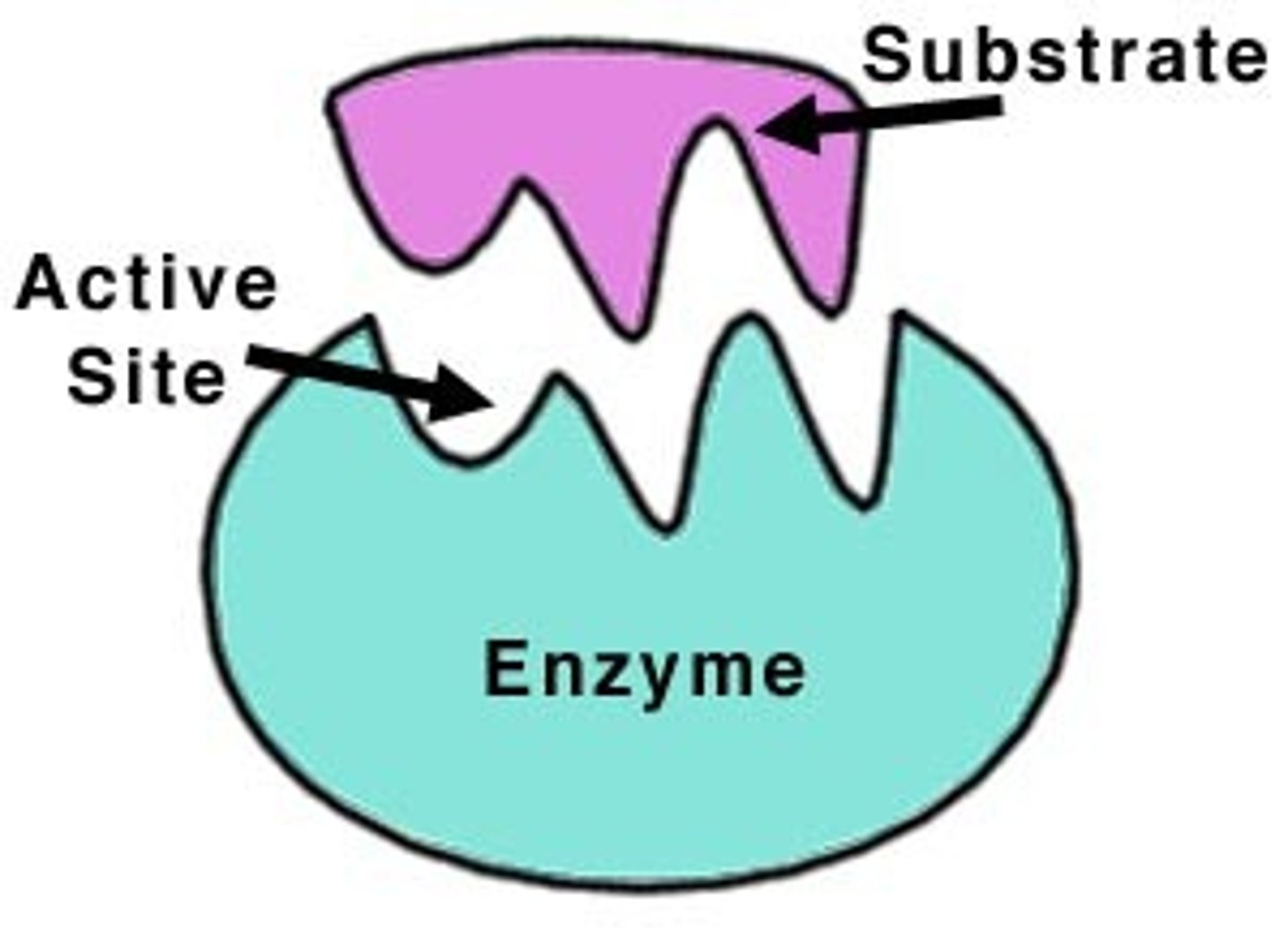
Substrate
A substance on which an enzyme acts during a chemical reaction.
Catalyst
A substance that speeds up the rate of a chemical reaction by lowering the energy required for the reaction to occur
Enzyme
A protein that acts as a catalyst and speeds up chemical reactions in a living thing by lowering the energy needed to get the reaction going; not used up during a reaction
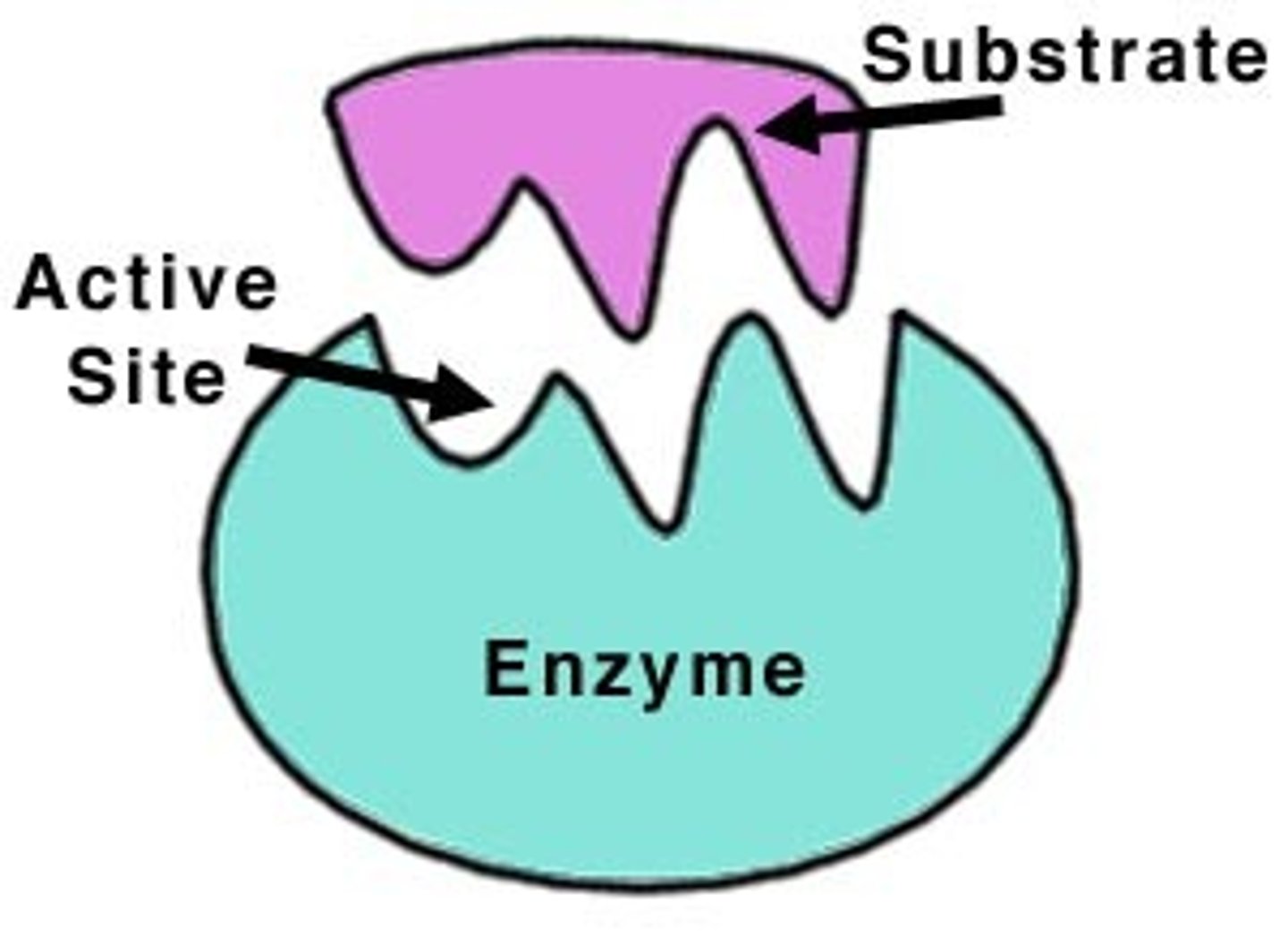
Enzymes (Naming)
The first part of an enzyme's name is usually derived from the substrate it catalyzes; the ending of the enzyme's name typically but not always ends in -ase
Enzyme (Roles)
Play a key role in:
- Regulating chemical pathways
- Making materials the cell needs
- Releasing energy
- Transferring information
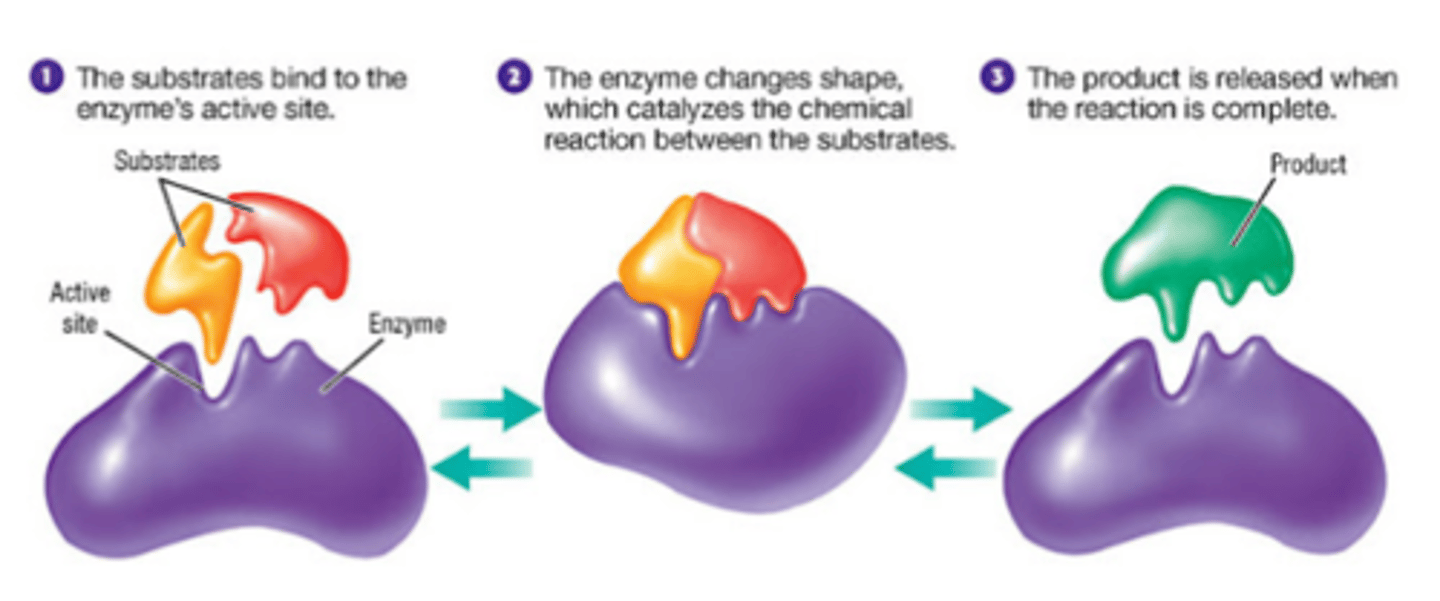
Active site
Location on the enzyme where the substrate will bind.
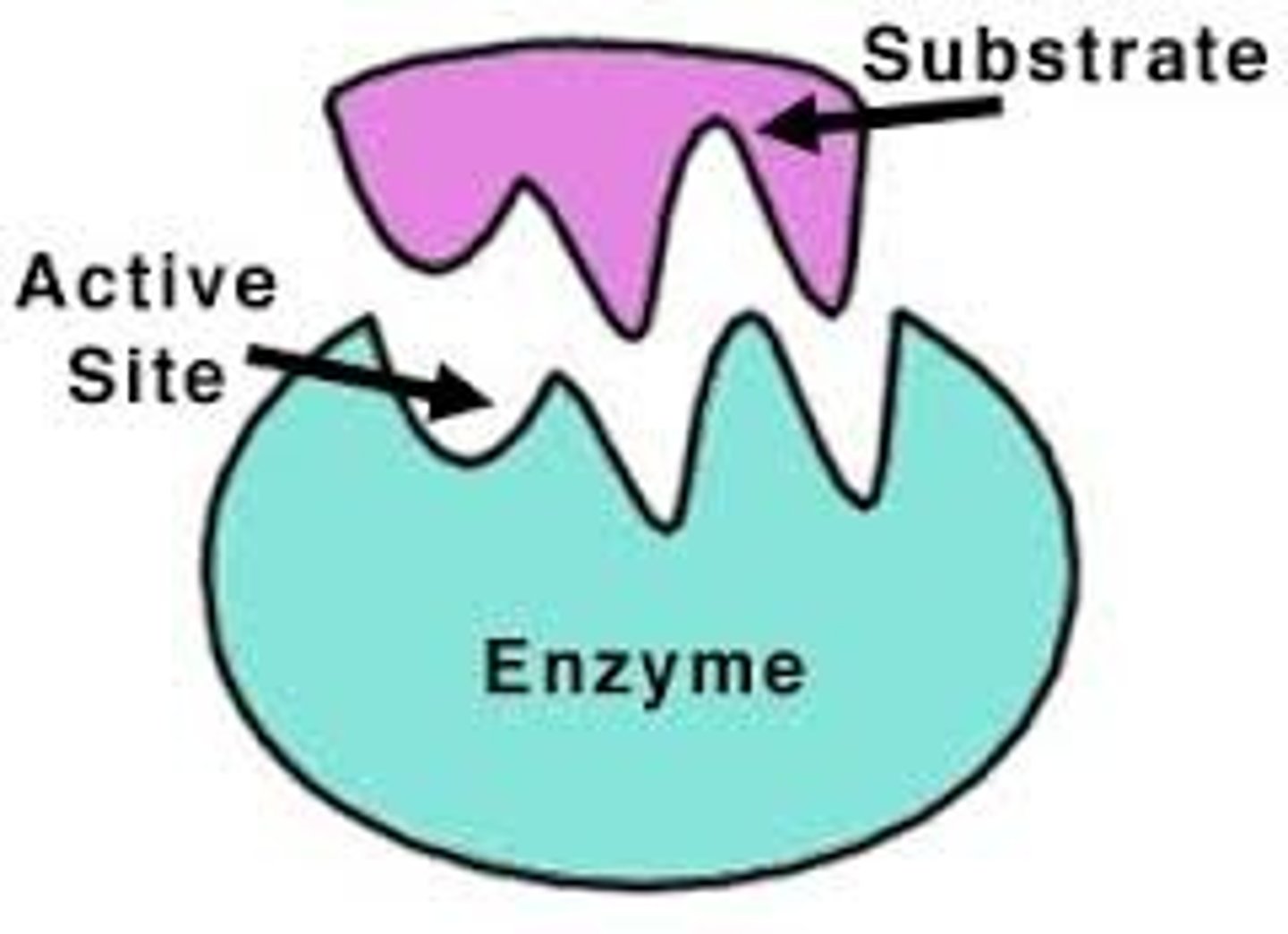
Enzyme-Substrate Complex
A temporary complex formed when an enzyme binds to its substrate molecule(s)
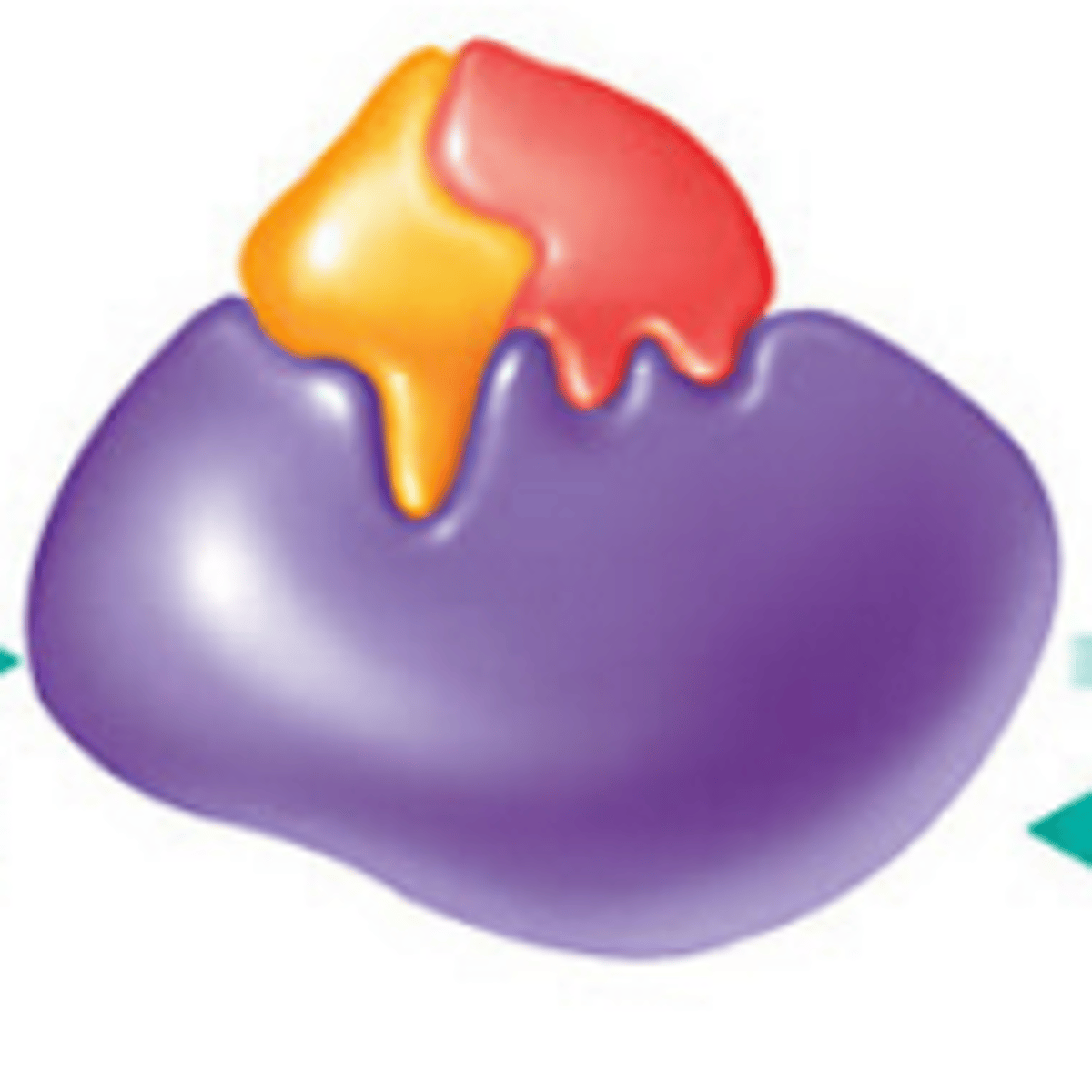
-ase
Ending on word typically indicates an enzyme
Denature
A change in the shape of a protein (such as an enzyme) that stops the protein from functioning. Can be caused by changes in conditions like temperature or pH
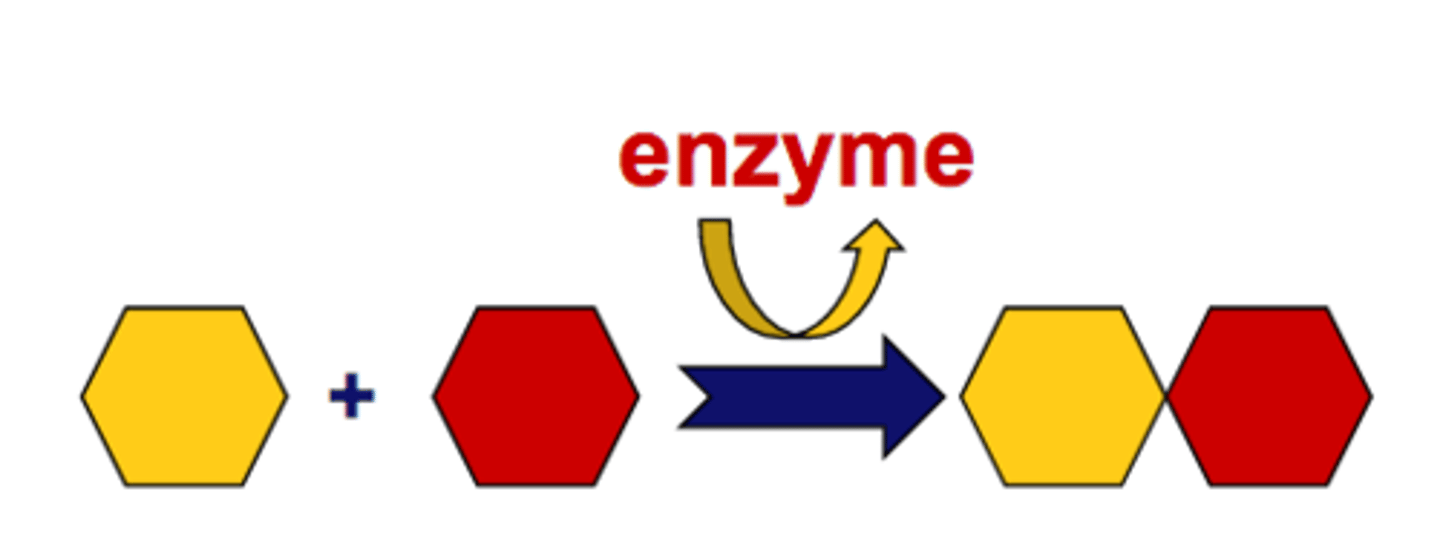
Synthesis Reaction
A reaction in which two or more substances combine to form a new compound
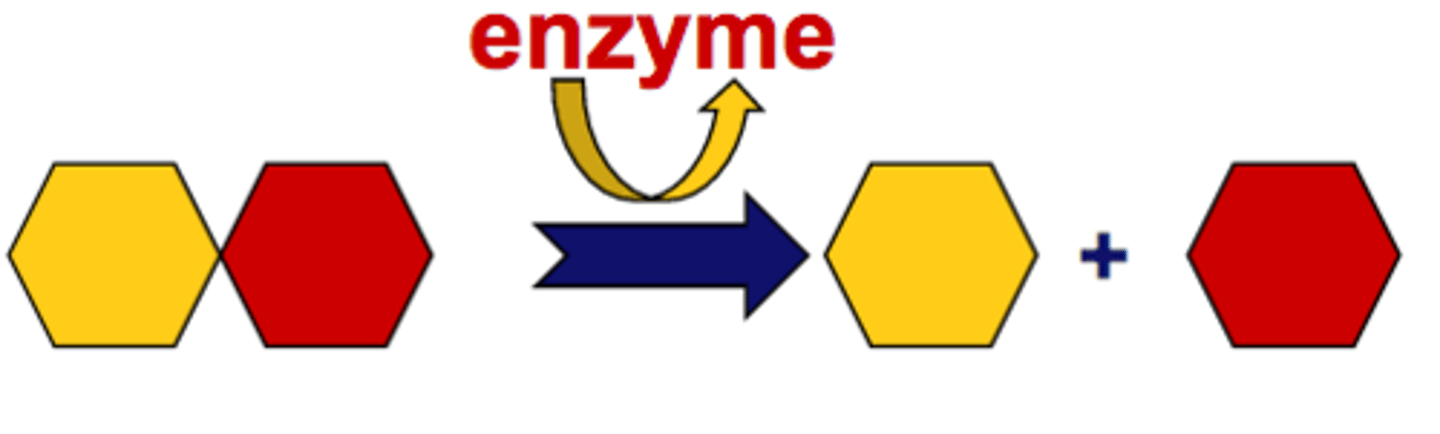
Decomposition Reaction
A reaction in which a single compound breaks down to form two or more simpler substances
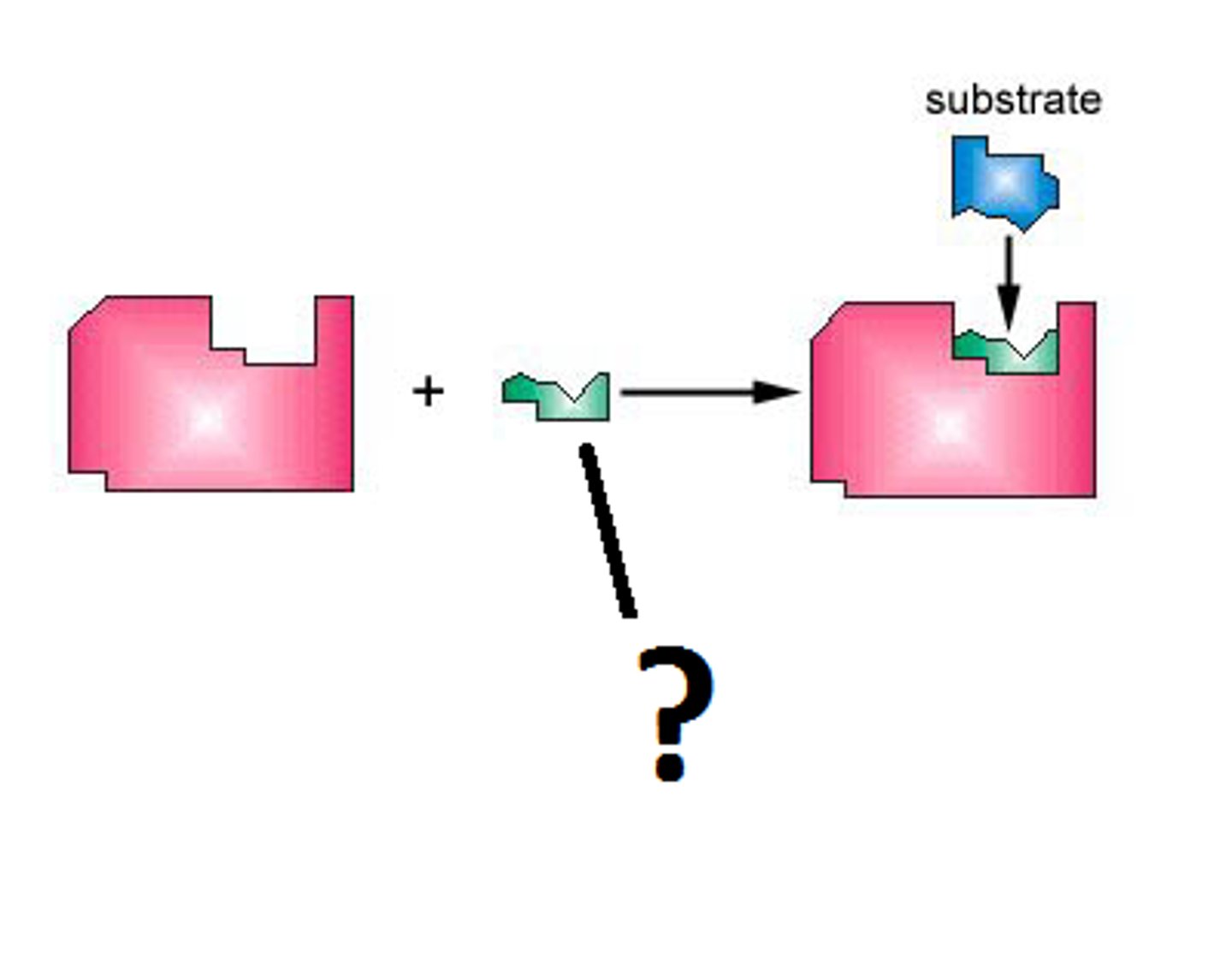
Cofactor
Non-protein molecules that help the enzyme work
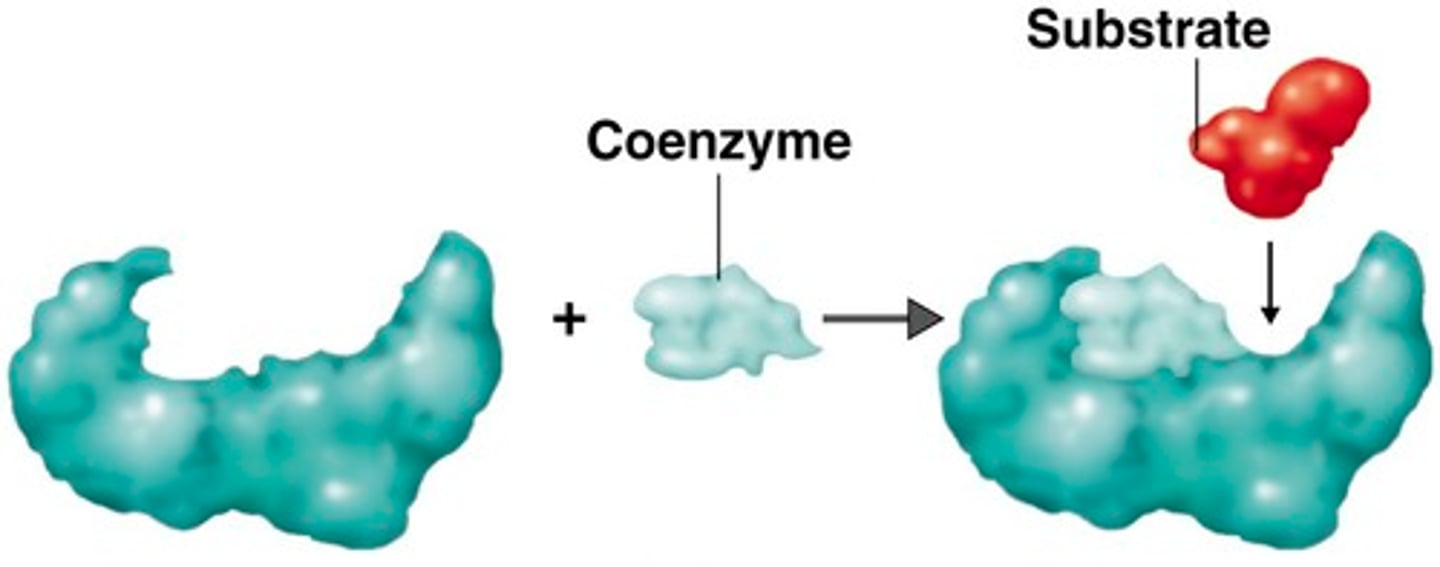
Coenzymes
An organic molecule that is a necessary participant in some enzymatic reactions, examples include some vitamins, ATP, NAD+
Enzyme (Action)
Enzymes bind to their substrates and bring them together in the proper orientation for the reaction to begin; the binding and aligning of the substrates lowers the activation energy of the reaction; the product is released when the reaction is complete
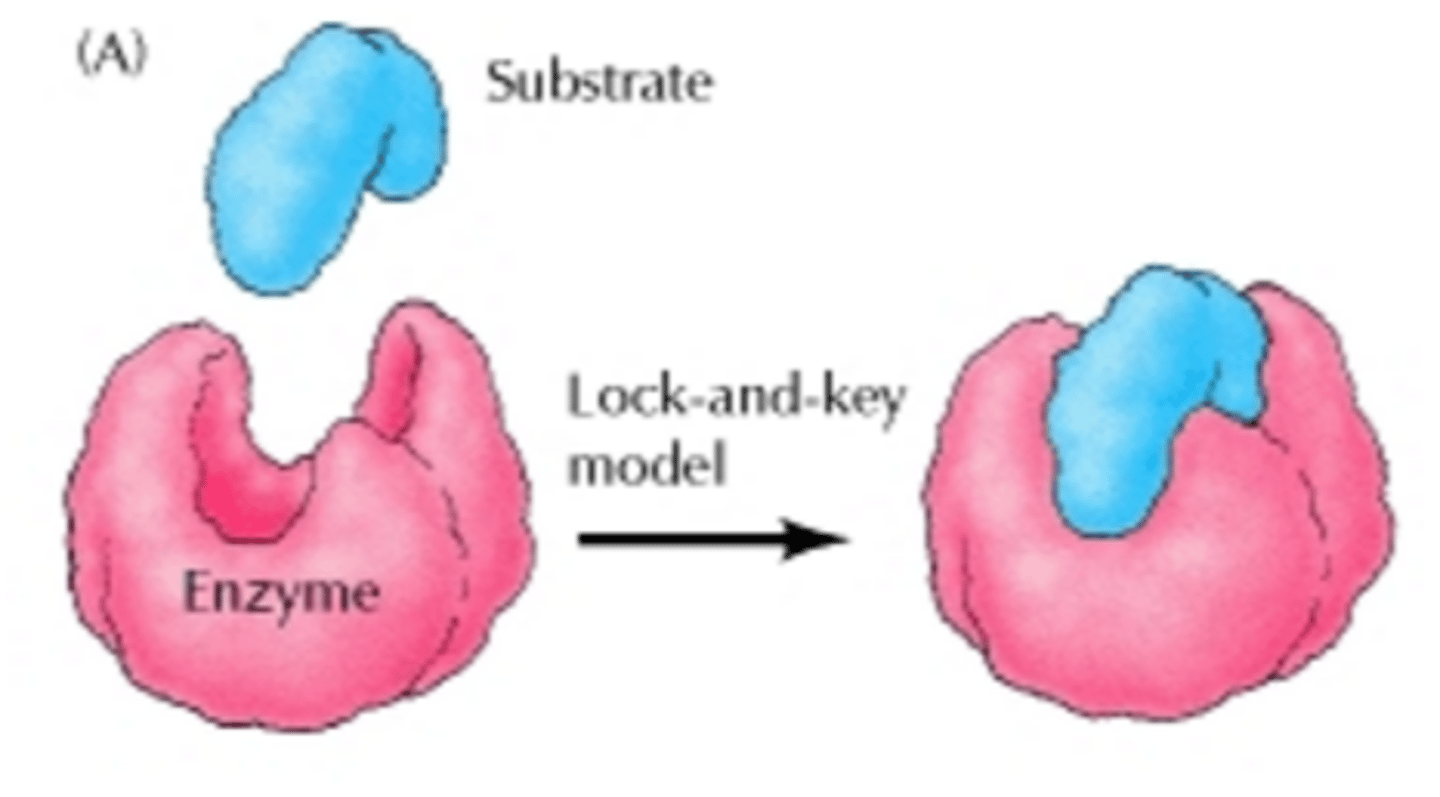
Enzyme Activity (Lock and Key Model)
Proposed model of enzyme activity in which the substrate and the enzyme are complementary to one another and fit perfectly like a key into a lock
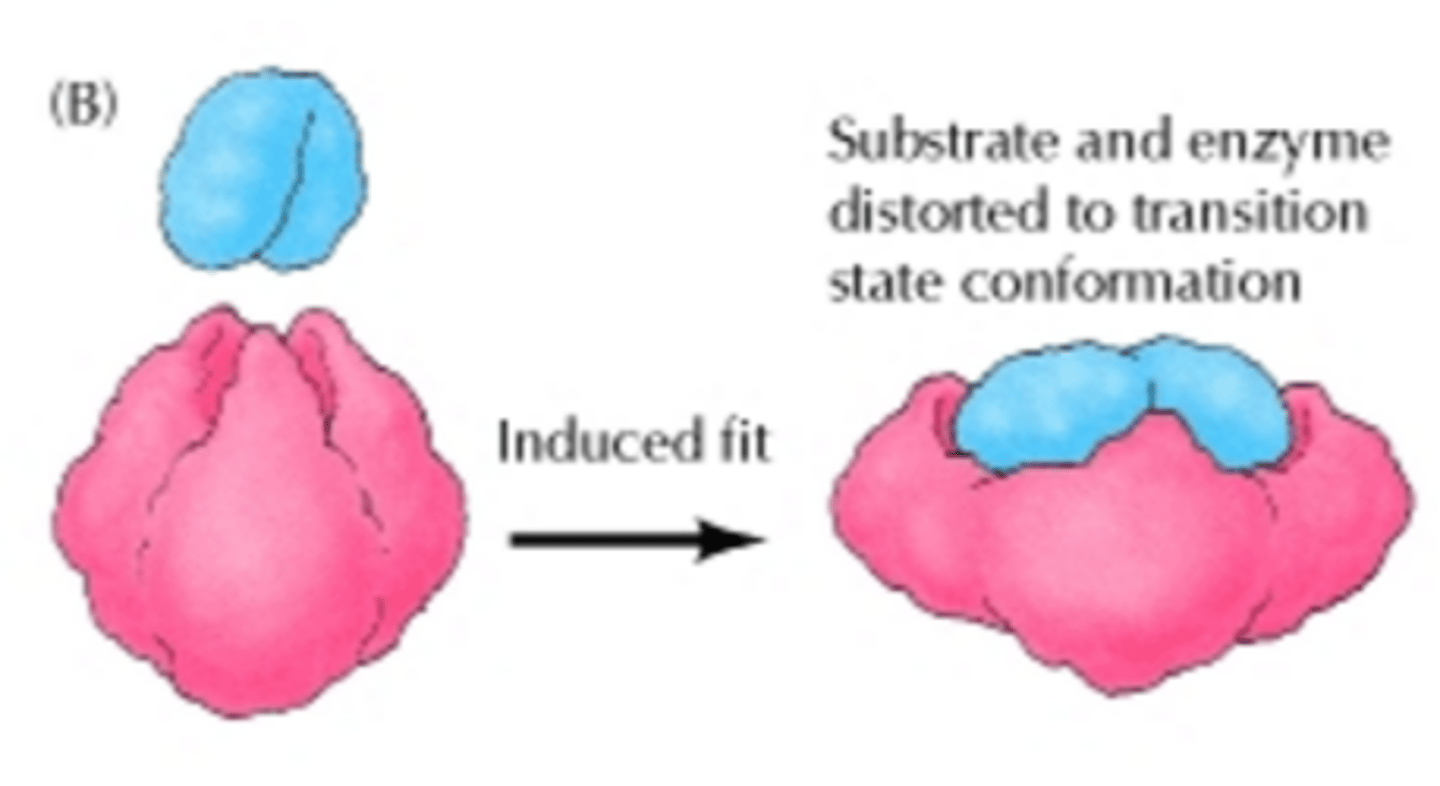
Enzyme Activity (Induced Fit Model)
Proposed model of enzyme activity in which the enzyme must undergo a conformational change upon binding to the substrate before the shape of the active site and enzyme become complementary to one another.
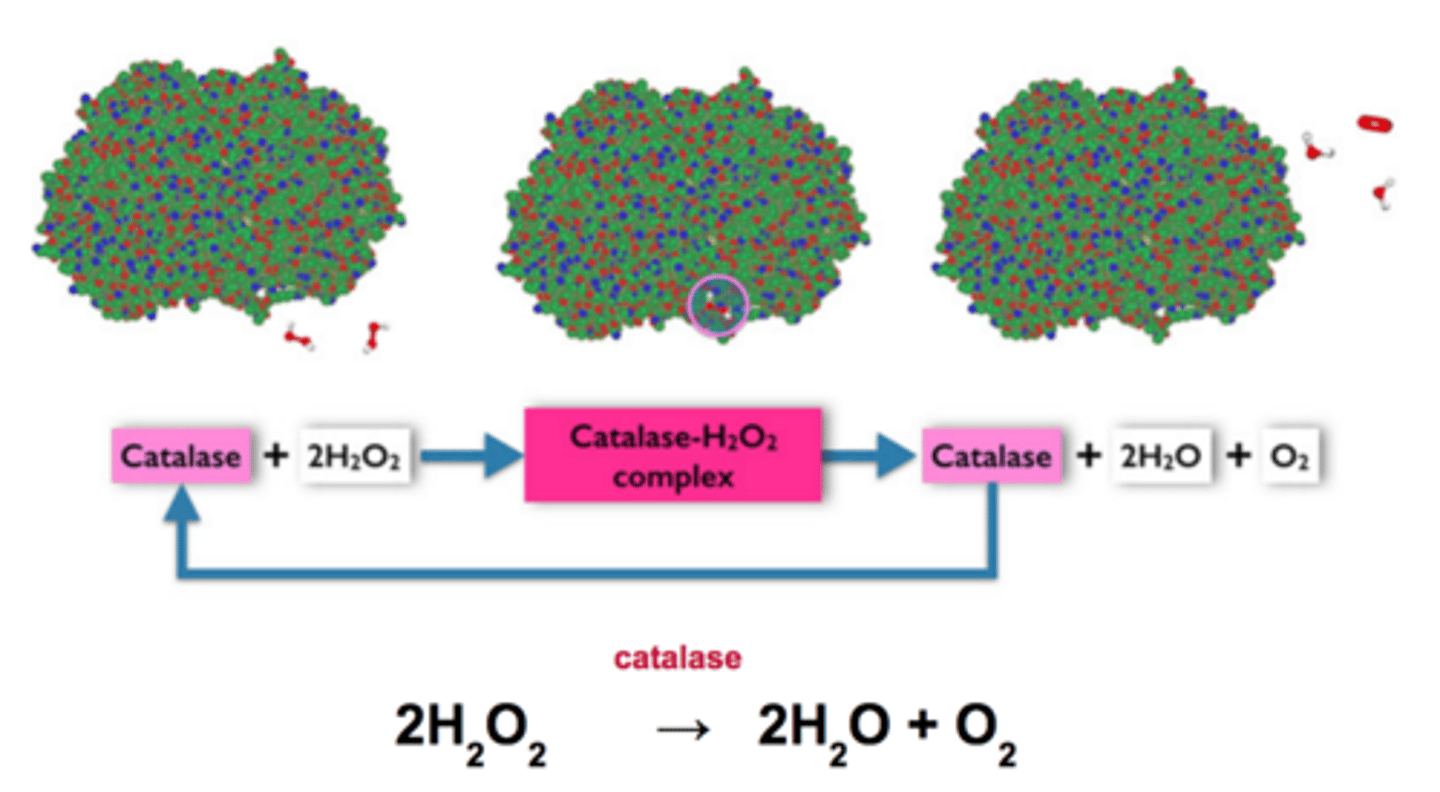
Enzyme catalyzed Reaction (Example)
One molecule of catalase can break 40 million molecules of hydrogen peroxide each second
Factor Affecting Enzyme Action
Enzyme/Substrate Concentration
pH
Temperature
Inhibitors
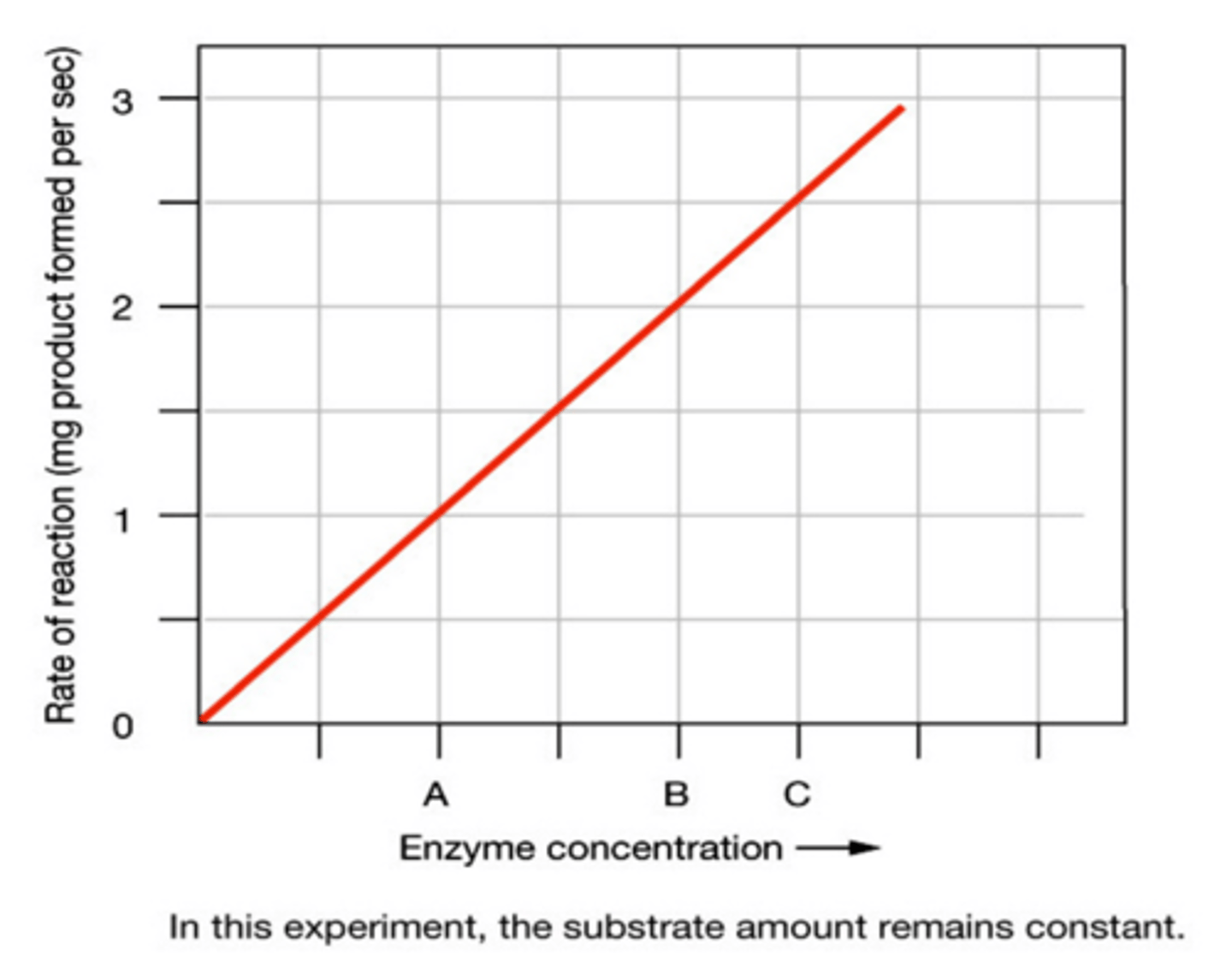
Enzyme Activity (Enzyme Concentration)
As enzyme concentration increases and the substrate concentration stays the same the number of active sites exceeds the number of substrate molecules so the rate of reaction will increase.
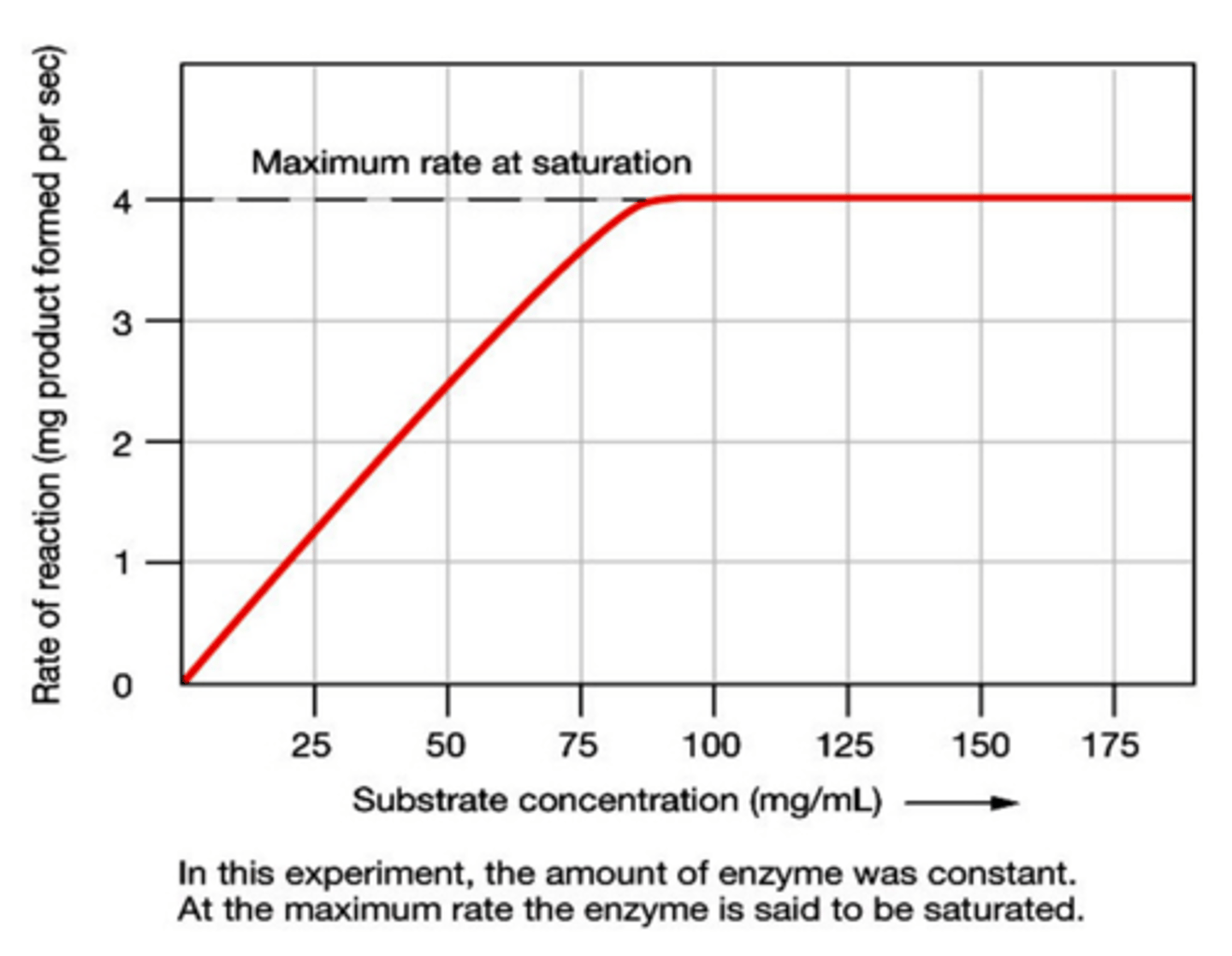
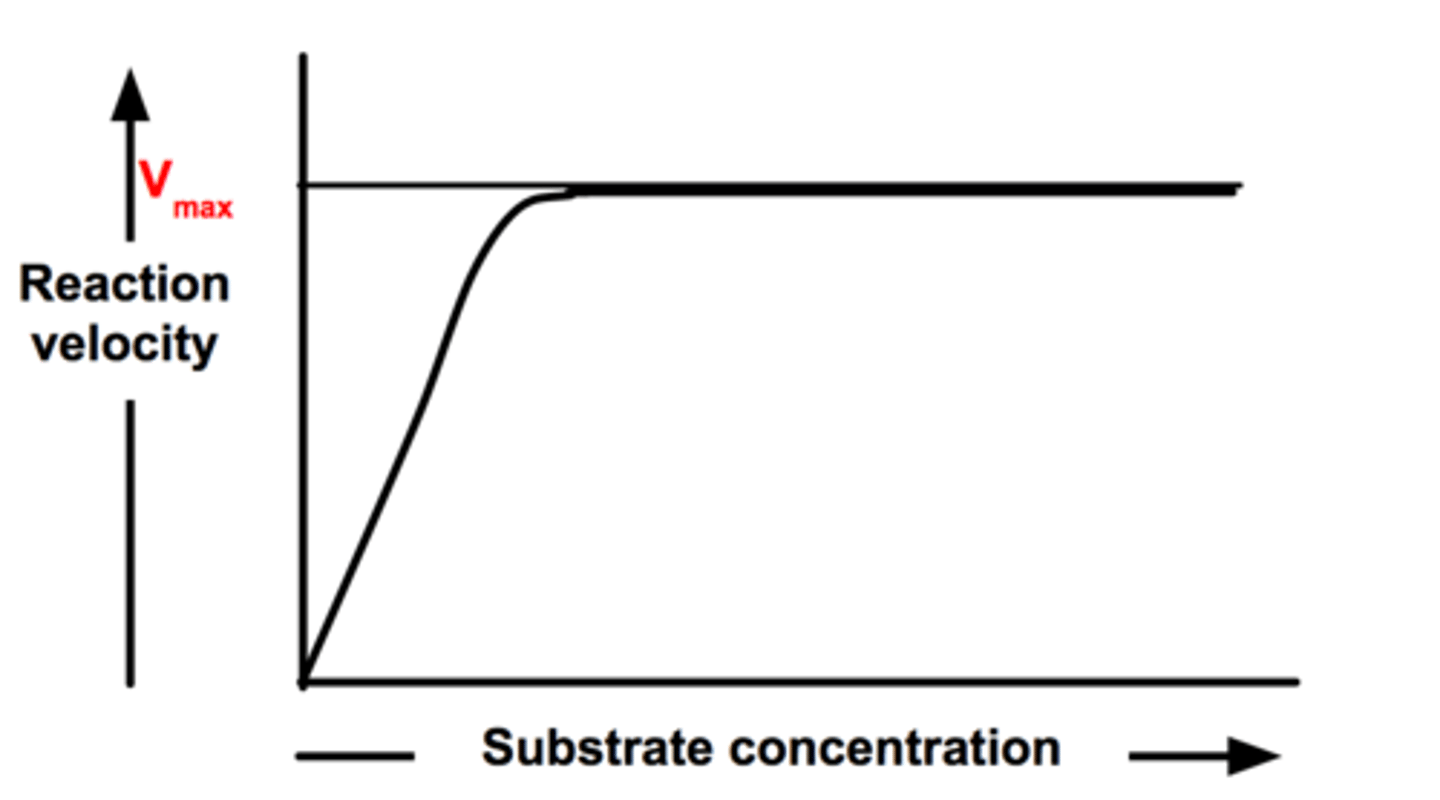
Enzyme Activity (Substrate Concentration)
As substrate concentration increases the rate of the chemical reaction will increase until the number of substrate molecules exceeds the number of enzyme active sites at which point the active sites become saturated and the rate of the reaction will level off
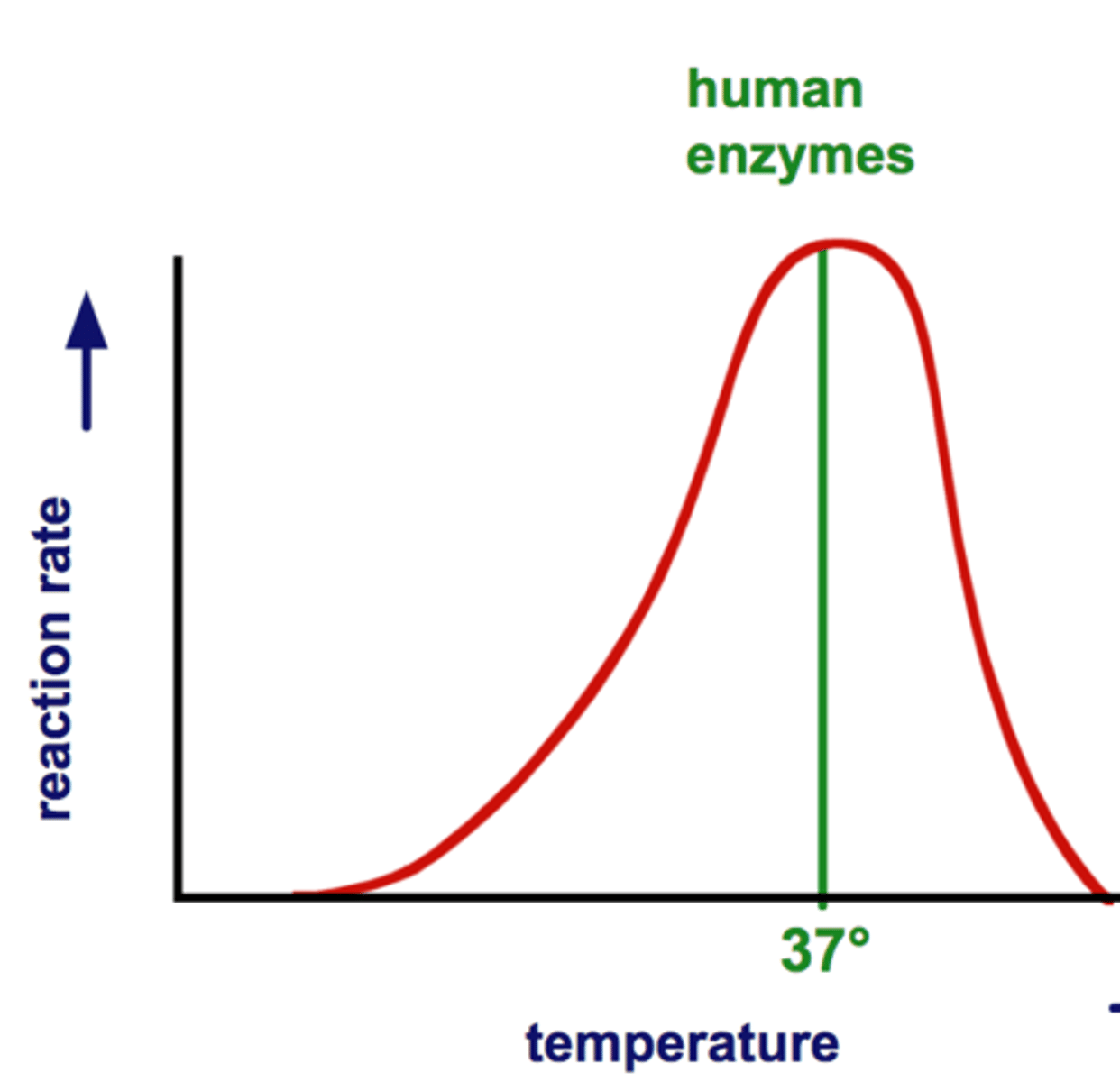
Enzyme Activity (Effect of Temperature)
Enzymes typically have optimum temperature at which the greatest number of collisions occur between enzyme & substrate; beyond a certain temperature the heat will break bonds in the enzyme and denature the enzyme so the active site can no longer bind with the substrate; as a result the rate of the reaction will decrease until the reaction can no longer occur
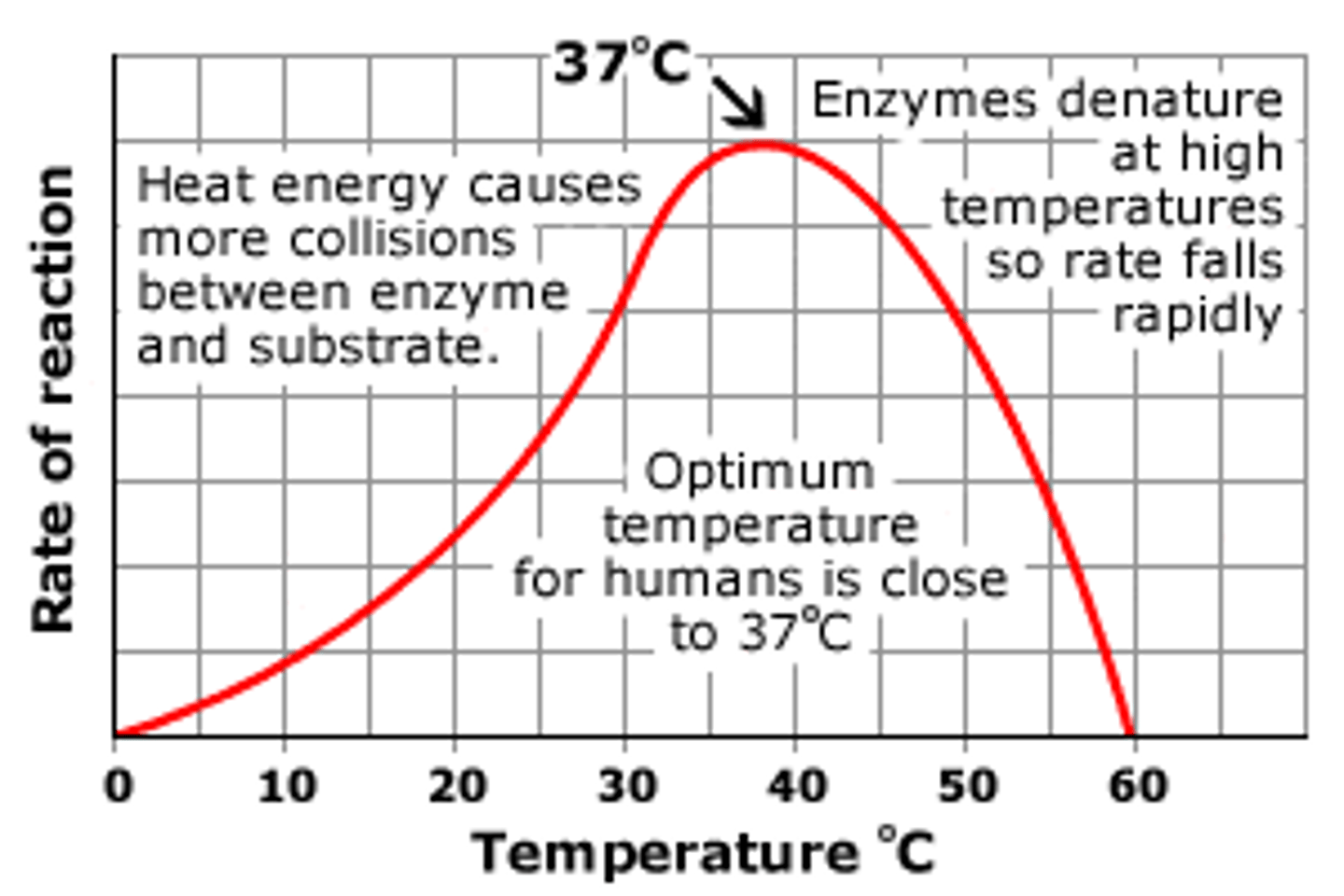
Optimum temperature (Description)
The temperature at which the greatest number of collisions occur between enzyme & substrate; varies for each enzyme
Optimum temperature (Examples)
Cold water fish will die at 30°C because their enzymes denature; a few bacteria have enzymes that can withstand very high temperatures up to 100°C; most enzymes however are fully denatured at 70°C
Enzyme Activity (Effect of pH)
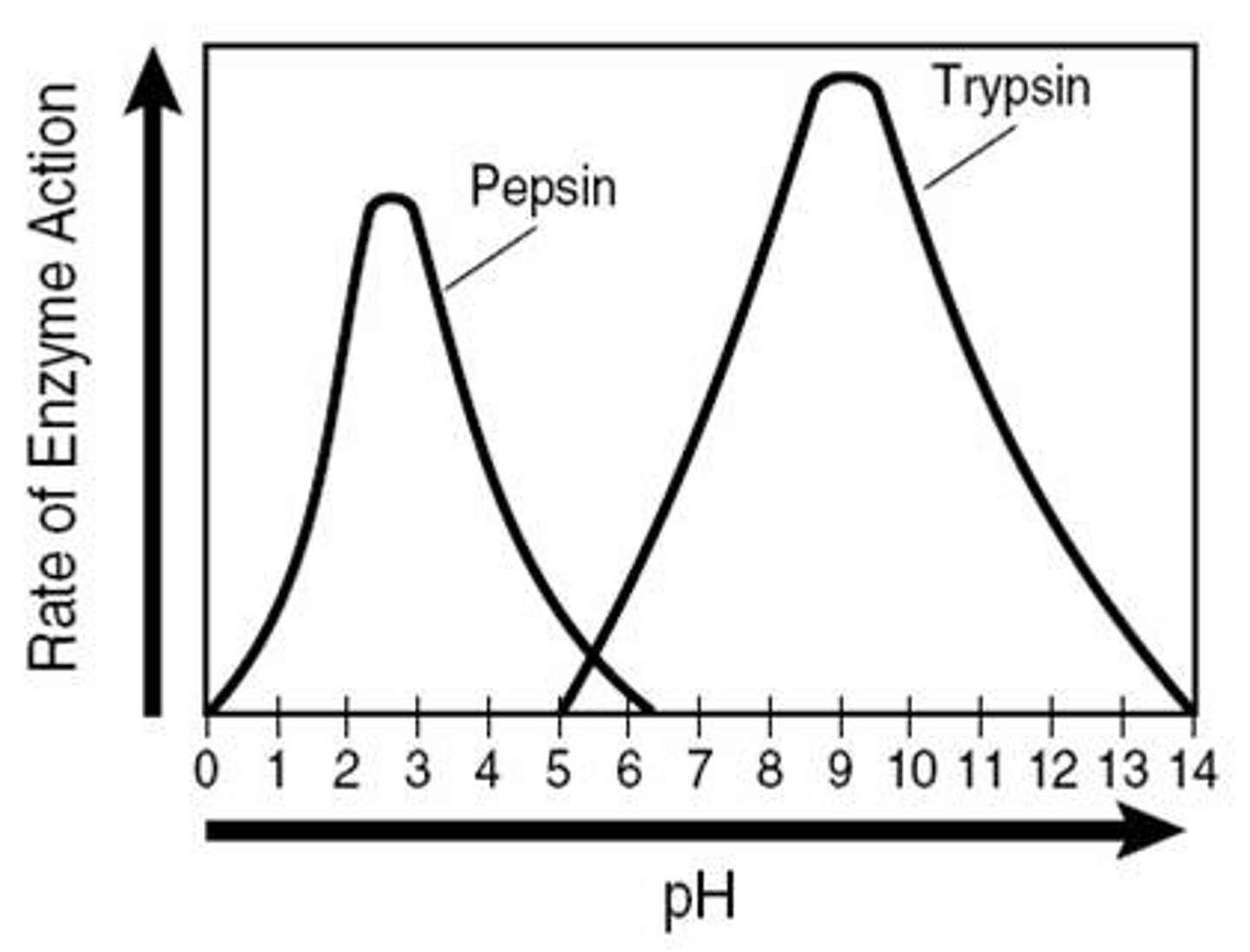
Enzyme typically operate best at an optimum pH; if the pH is too high or too low the enzymes will be denatured; most enzymes in the human body work best at a pH of 7; some enzymes, like protease in our stomach, work best at acidic conditions (ph 2)
Inhibitor
Chemicals that reduce the rate of reaction
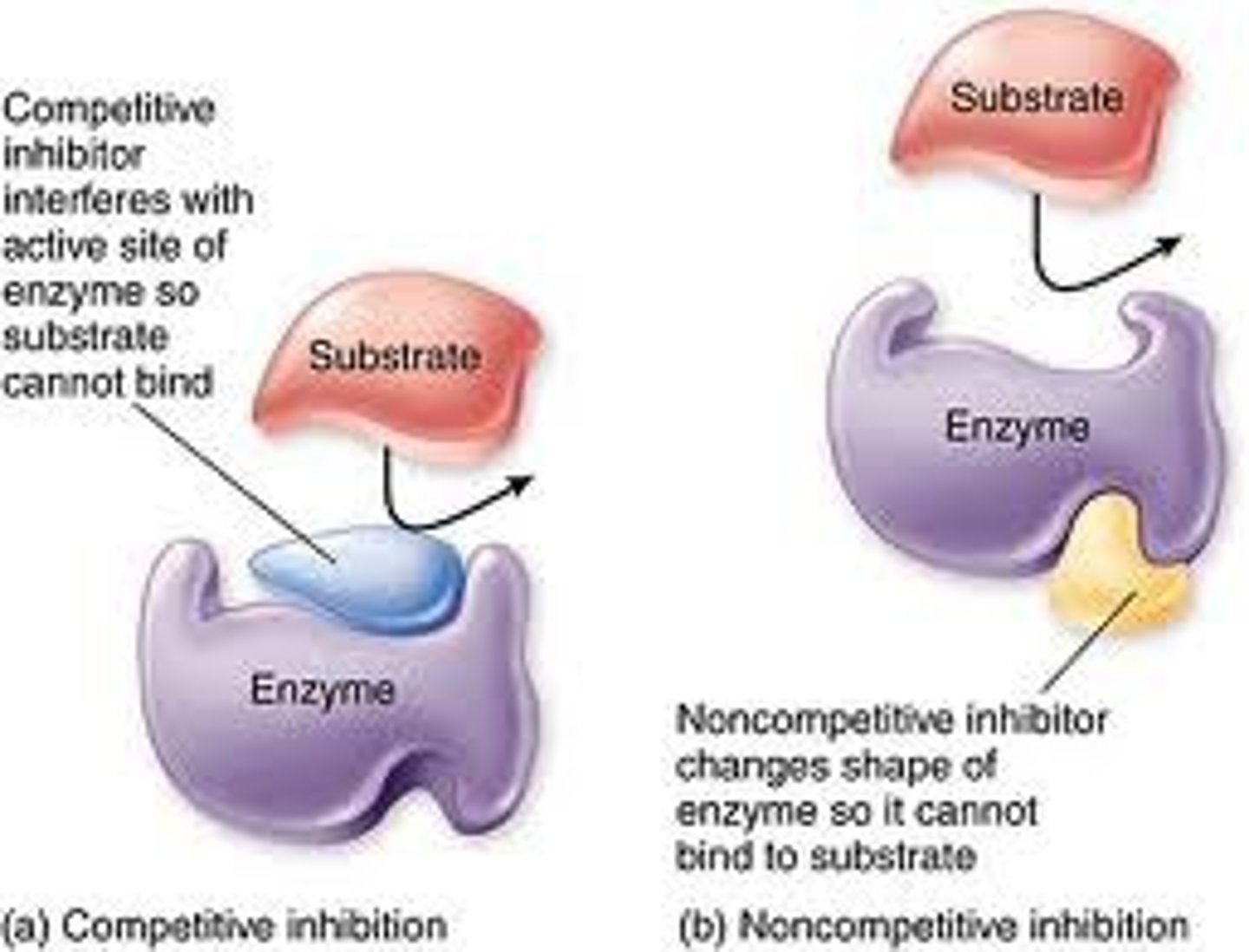
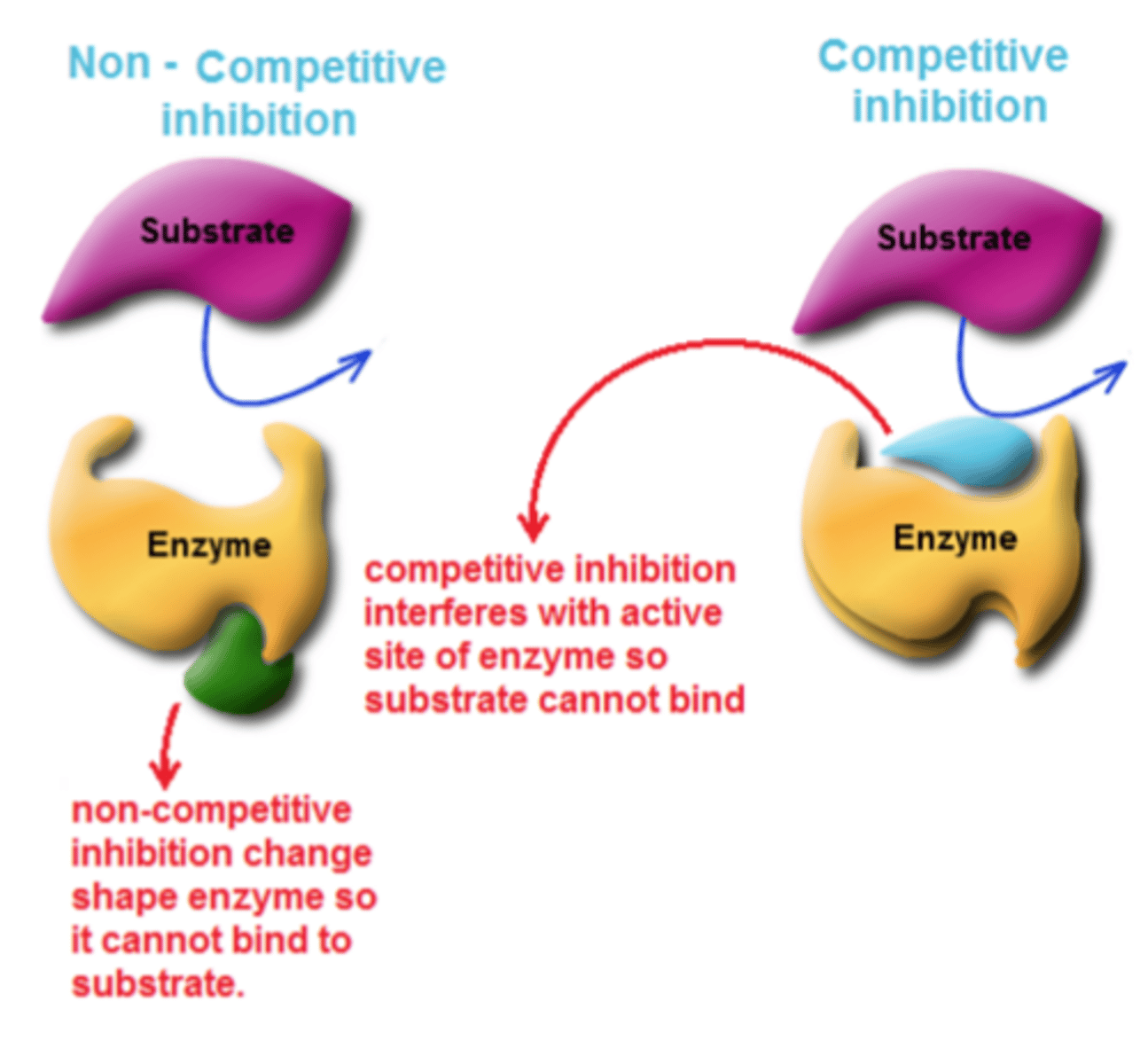
Competitive inhibition
Interferes with the active site of the enzyme but they do not usually destroy it
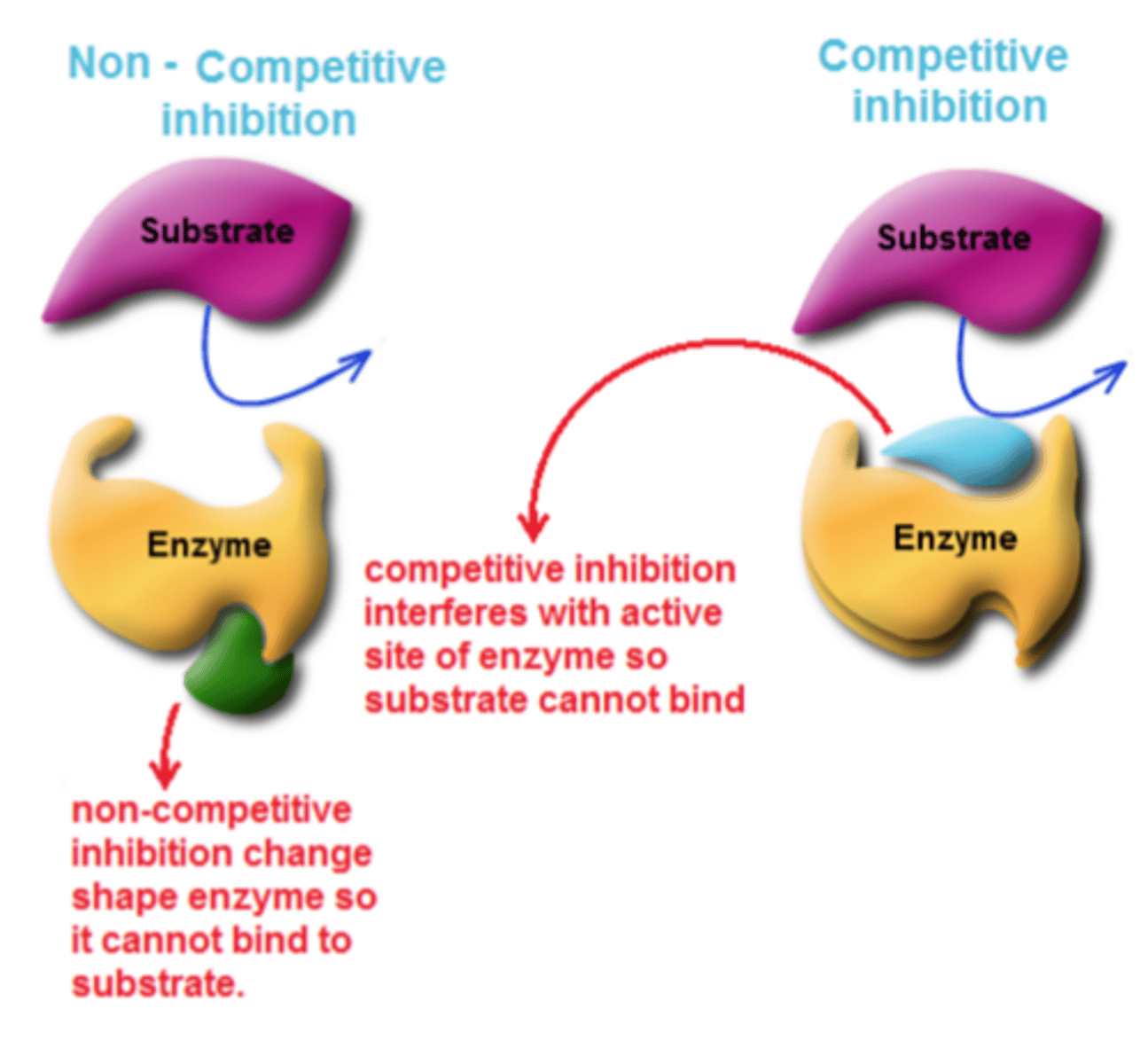
Non-competitive inhibition
Changes the shape of the enzyme so it can't bind with substrate
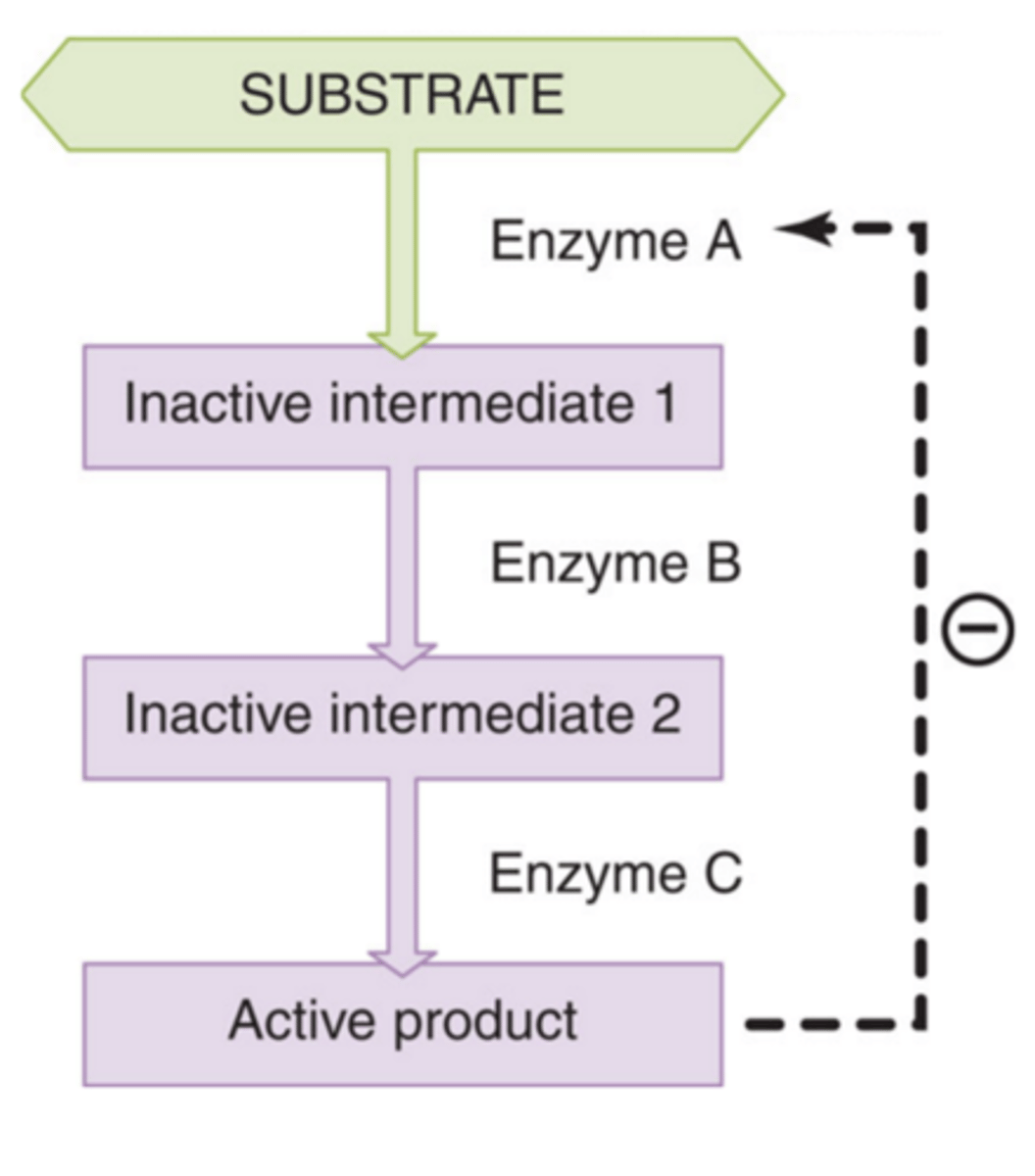
Feedback inhibition
Occurs when the product of a series of enzymatic reactions begins to accumulate within the cell; the product may then inhibit the action of the first enzyme; further production of the enzyme is then halted
Sucrase
An enzyme that breaks down the substrate sucrose into glucose and fructose
Lactase
An enzyme that breaks down the substrate lactose into glucose and galactose
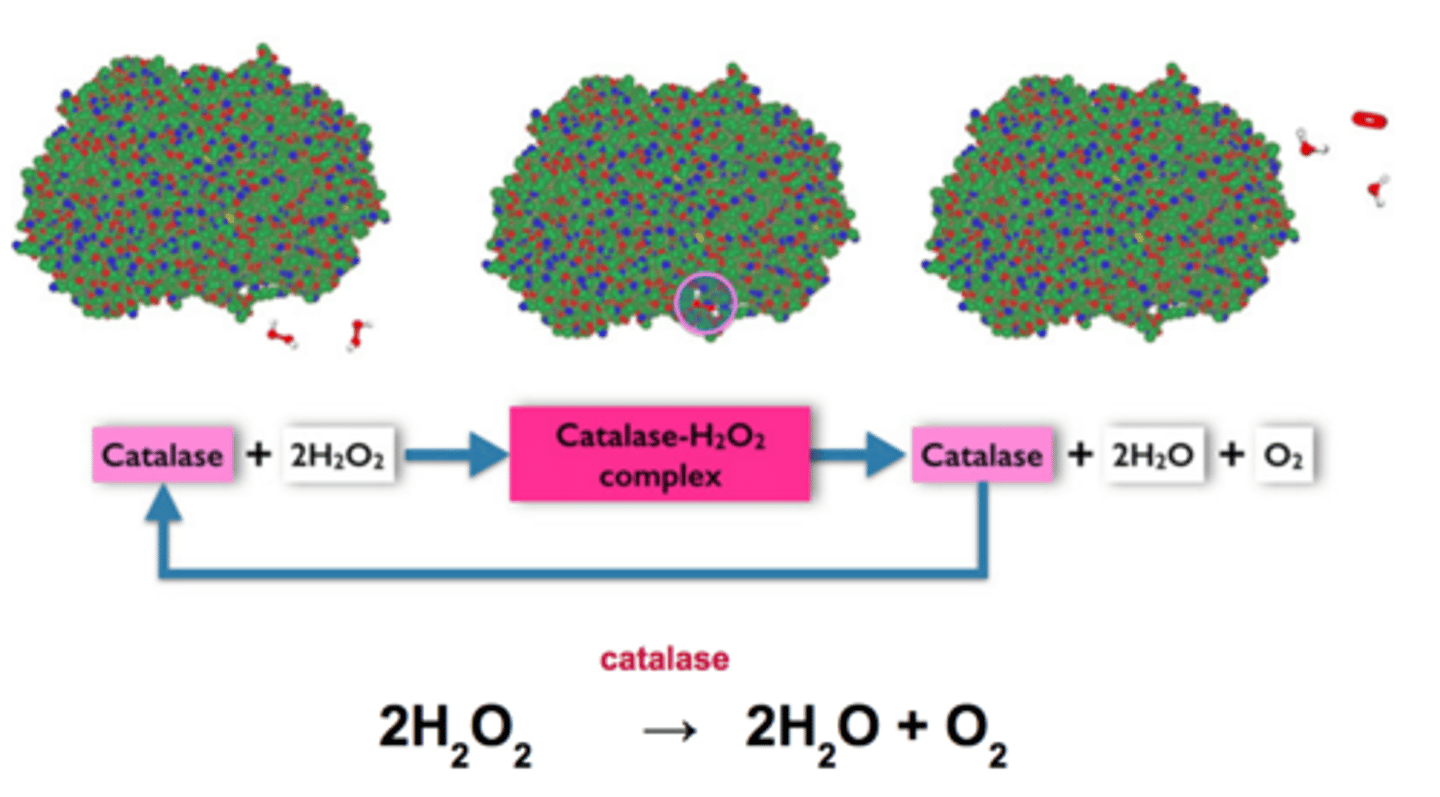
Catalase
An enzyme found in peroxisomes that breaks down the substrate hydrogen peroxide into water and oxygen
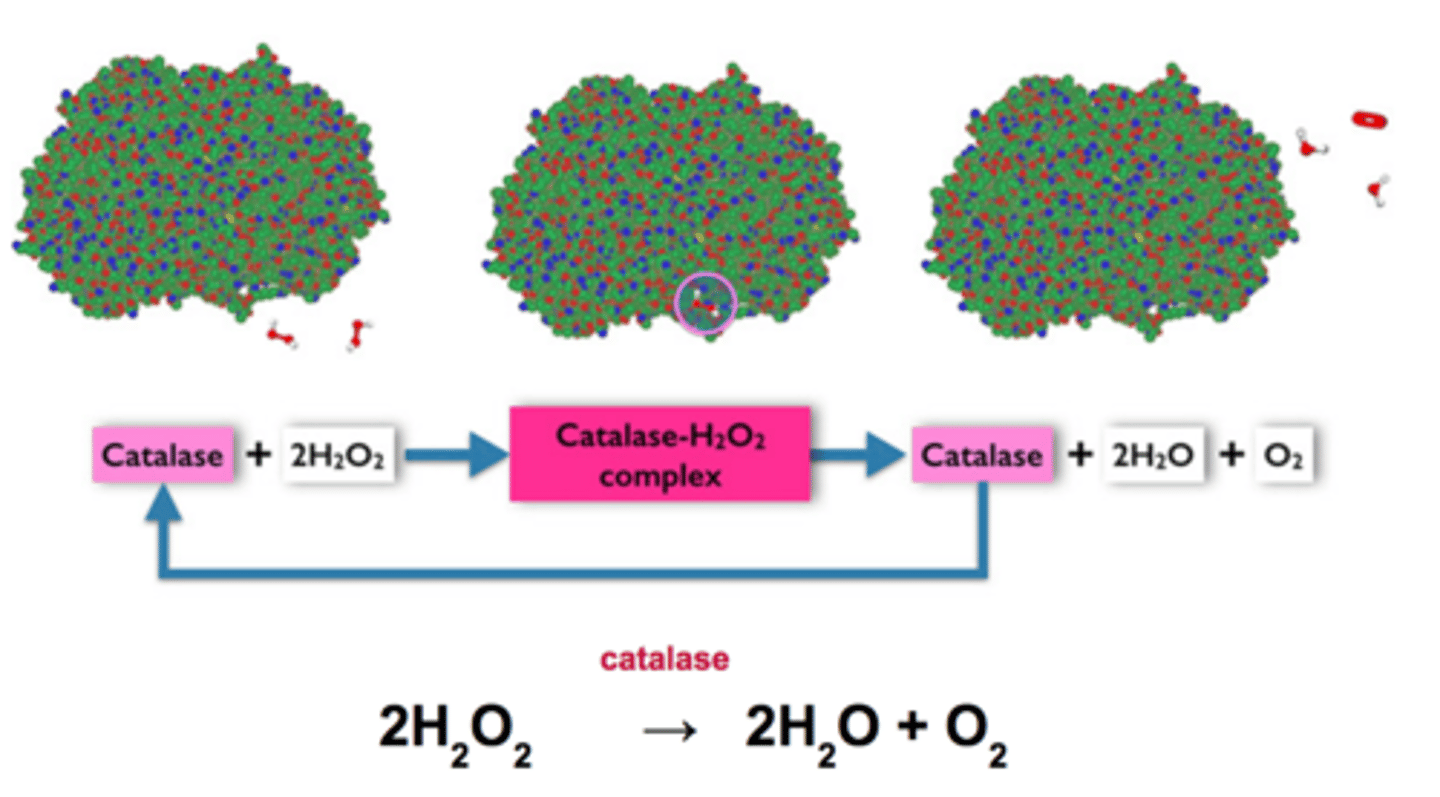
Hydrogen peroxide
A bi-product of cellular reactions that is toxic to cells
Inhibitors (Examples)
Examples include drugs and poisons
Saturation
The condition in which the addition of substrate exceeds the # of active sites causing the production of products to level off
Optimum pH (Examples)
Most human enzymes function best at a pH between 6-8
Depends on where the enzyme is found in body
Pepsin (stomach) = pH 2.5
Trypsin (small intestines) = pH 8
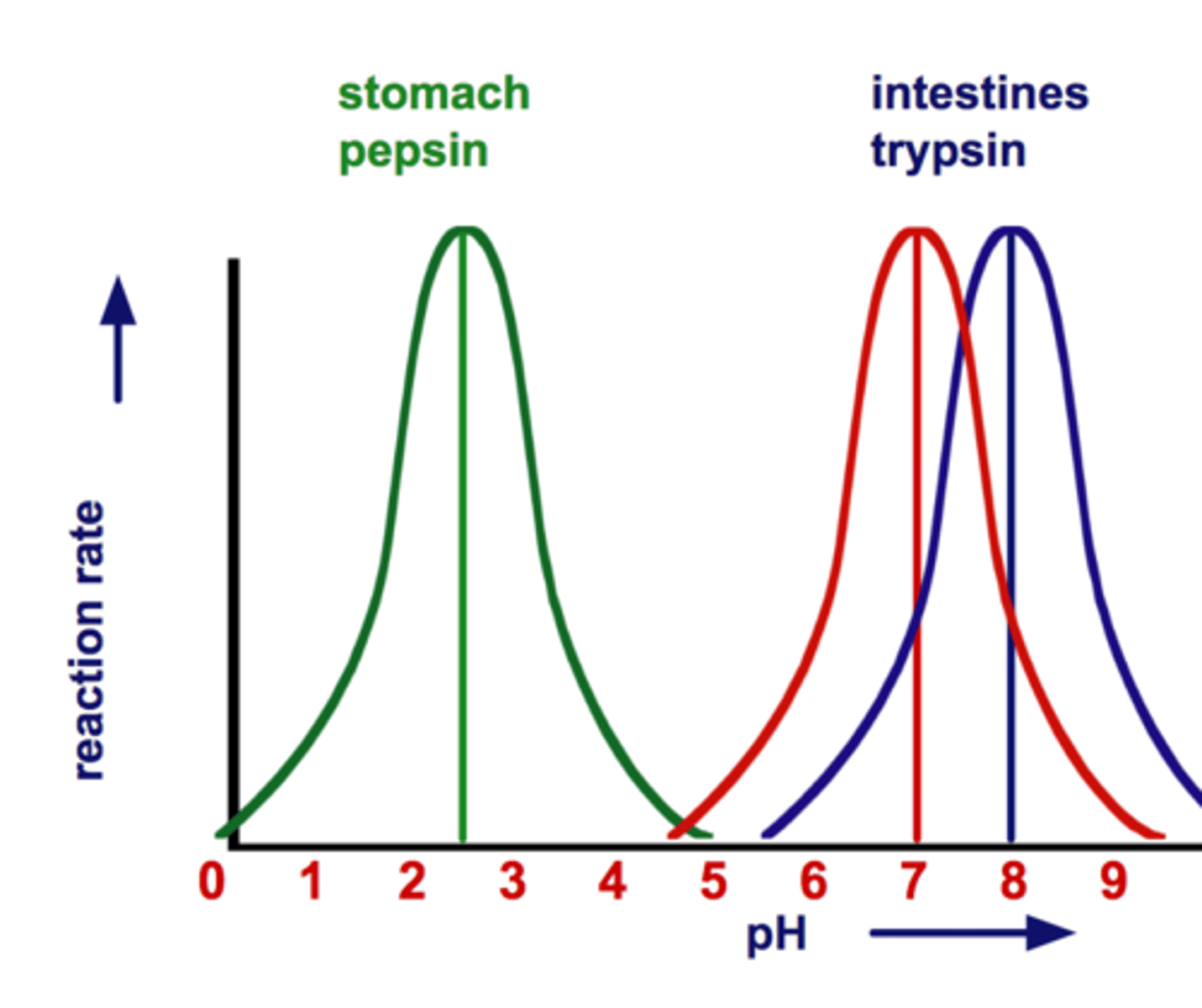
Optimum pH (Description)
The pH at which the greatest number of collisions occur between enzyme & substrate; varies for each enzyme
Optimum Temperature (Humans)
The optimum temperature for most enzymes is 35°- 40°C (body temp = 37°C) or 98.6 °F