ALL POST LABS
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
In the Diels-Alder experiment to synthesize 9,10-dihydroanthracene-9,10-a,b-succinct anhydride by reacting anthracene and maleic anhydride it was necessary to use a catalyst
(Lab 1: Diels-Alder)
false
If the diene is in s-trans conformation, the Diels-Alder reaction will be faster
(Lab 1: Diels-Alder)
False
Calculations of the yield can be affected by all of the following except:
(Lab 1: Diels-Alder)
The activation energy of the reaction
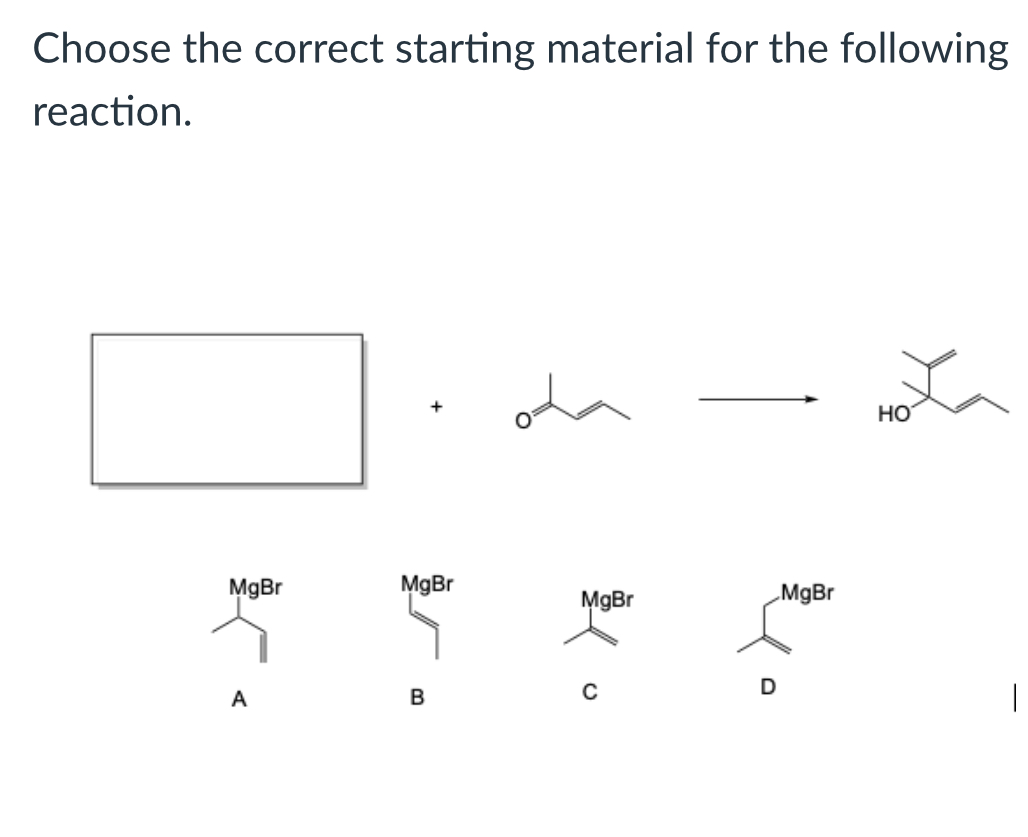
(Lab 2: grignard reaction)
C
How many signals do you expect to see in 13C NMR of your product?
(Lab 4: nitration of methyl benzoate)
8
Choose all the groups that can be classified as meta-directing groups in SeAr reactions
(Lab 4: nitration of methyl benzoate)
-CN
-NO2
-CO2CH2CH3
-N(CH2CH3)3
-CF3
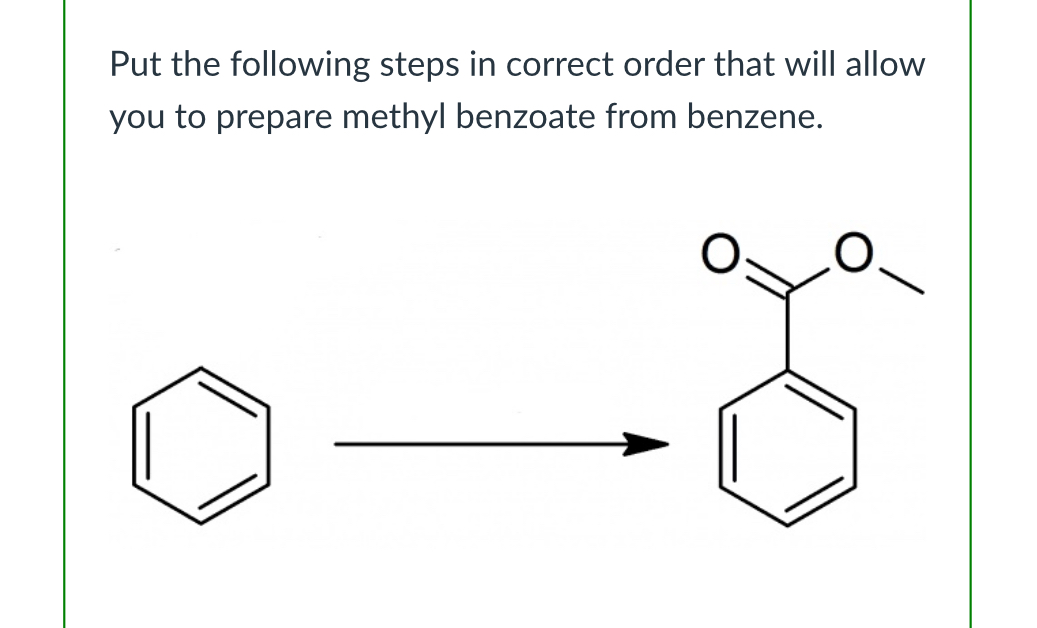
(Lab 4: nitration of methyl benzoate)
CH3Cl, AlCl3
KMnO4, H+, heat
CH3OH,H2SO4,heat

(Lab 3: Polymerization)
Condensation polymerization
Addition polymerization
Nylon is a polyamide polymer formed by reacting a dicarboxylic acid with a diamine
(Lab 3: Polymerization)
True
In a step growth polymerization, the growing chains may react with each other to form even longer chains while in chain growth polymerization, only monomers react with growing chains
(Lab 3: Polymerization)
True
Slime has gel-like consistency due to trapped molecules of water in the polymeric net structure
(Lab 3: Polymerization)
True
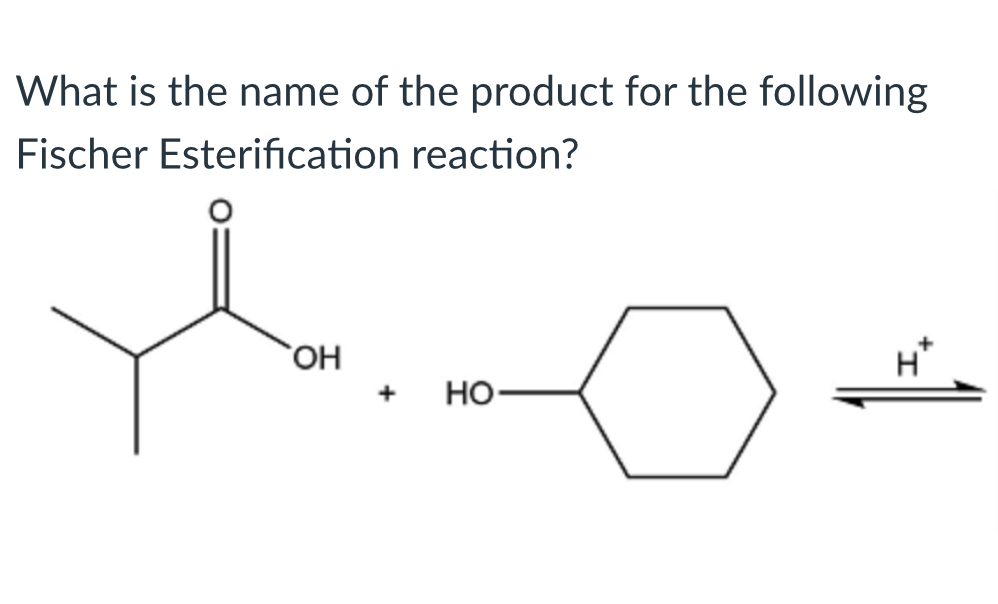
(Lab 5: Fischer esterification)
Cyclohexyl isobutanoate
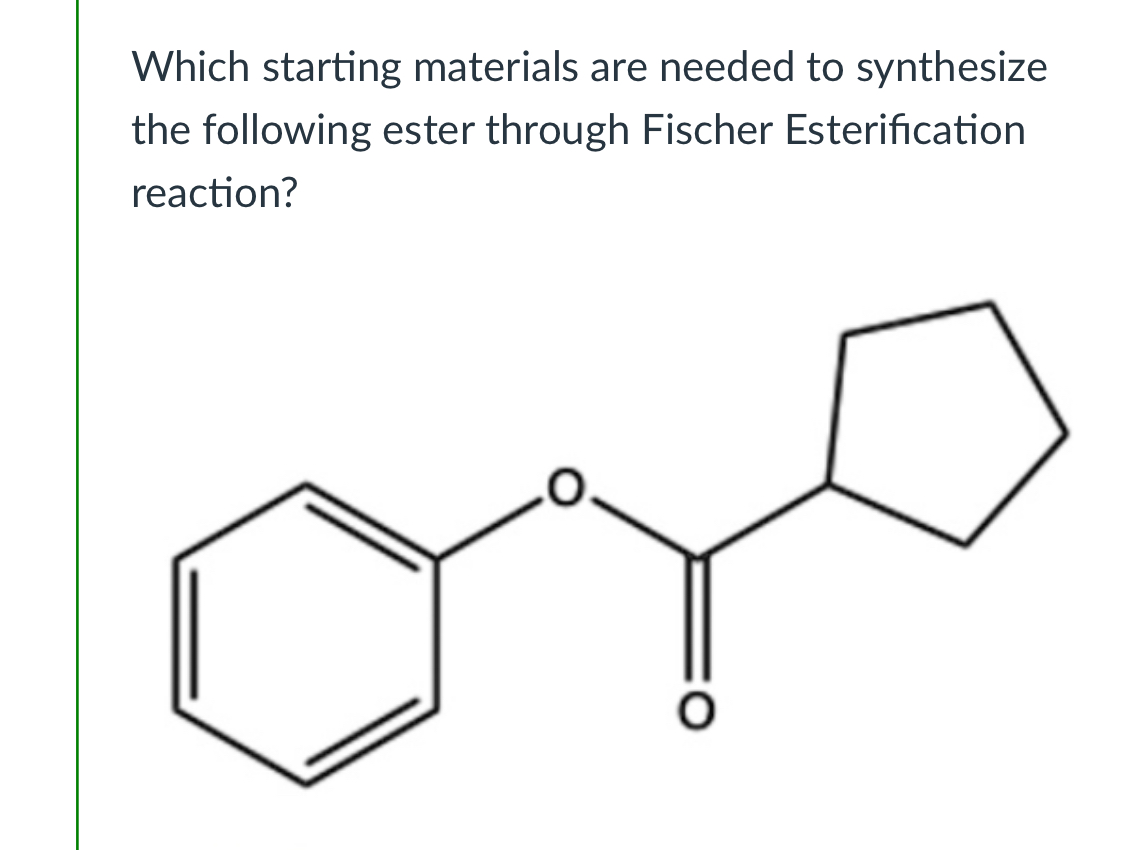
(Lab 5: Fischer esterification)
Phenol + cyclopentanoic acid
Aspirin can react with water under acidic conditions and it will hydrolyze back into salicylic acid
(Lab 6: Aspirin)
True
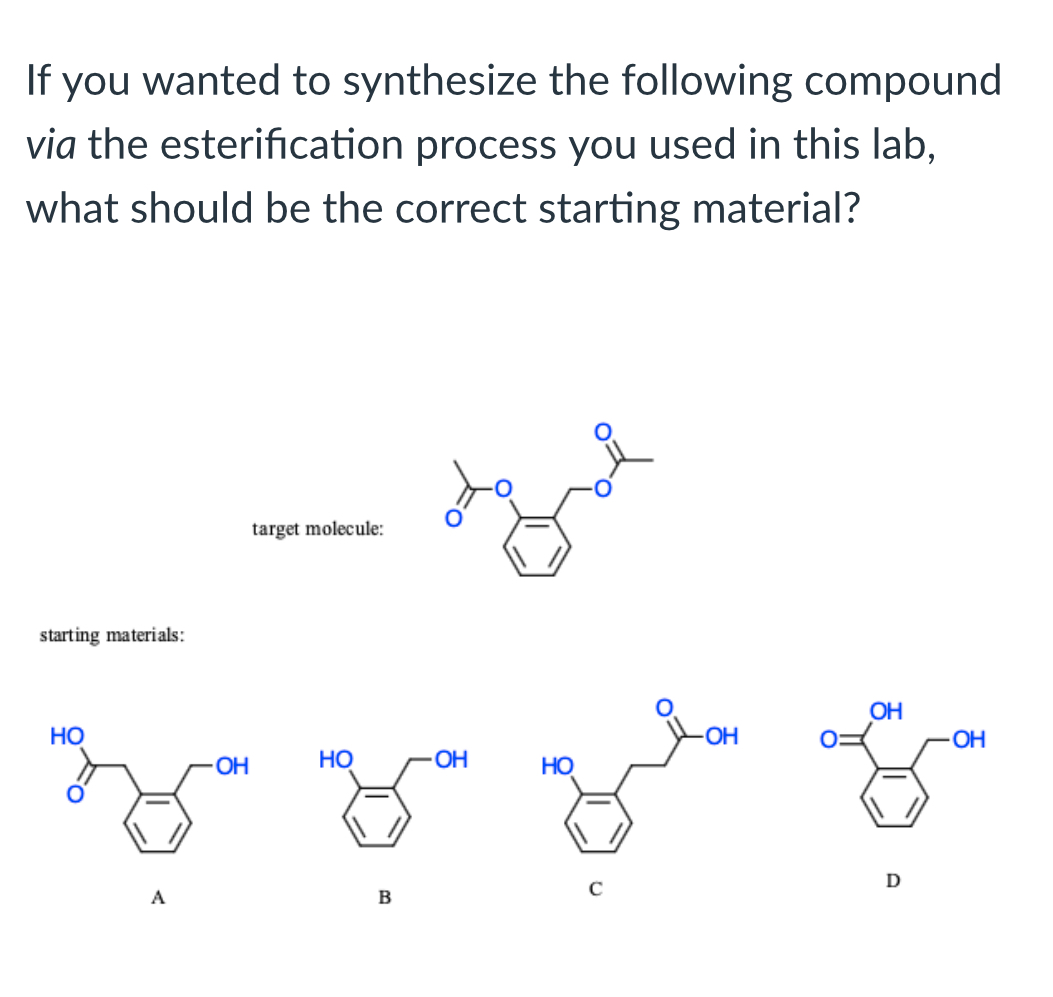
(Lab 6: Aspirin)
B
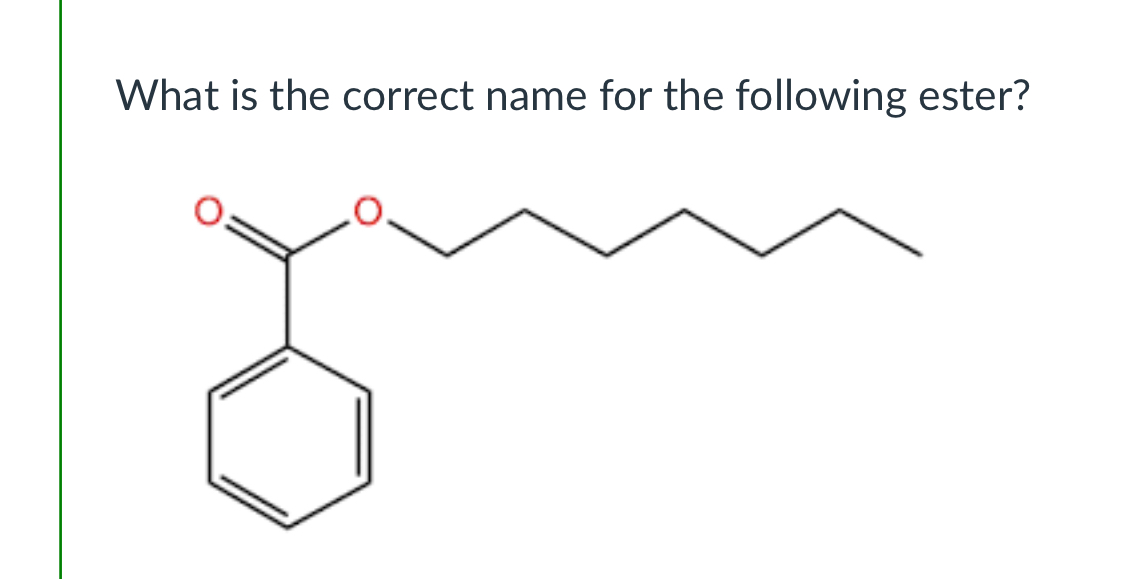
(Lab 6: Aspirin)
heptyl benzoate
What was the main purpose of using TLC in this process?
(Lab 7: Wittig reaction)
To monitor the progress of the reaction
Based on your TLC sketch and calculated Rf values, how does polarity of your product compares to the polarity of cinnamaldehyde?
(Lab 7: Wittig reaction)
Product is less polar than cinnamaldehyde because Rf of the product is larger than Rf of the cinnamaldehyde
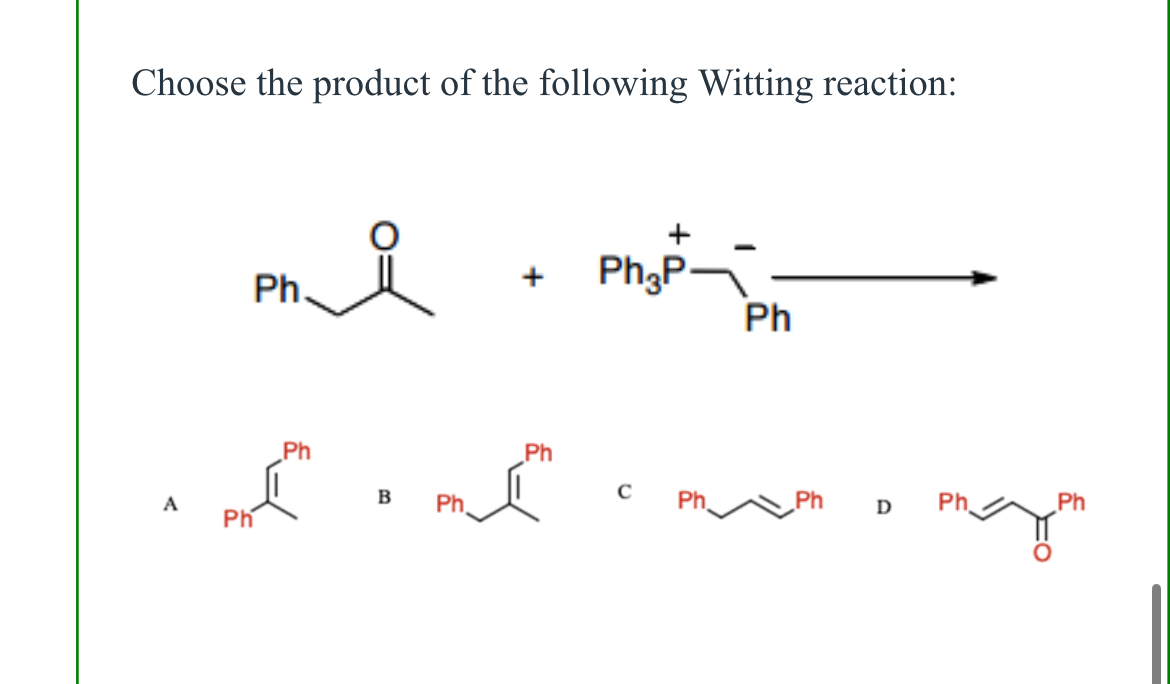
(Lab 7: Wittig reaction)
B
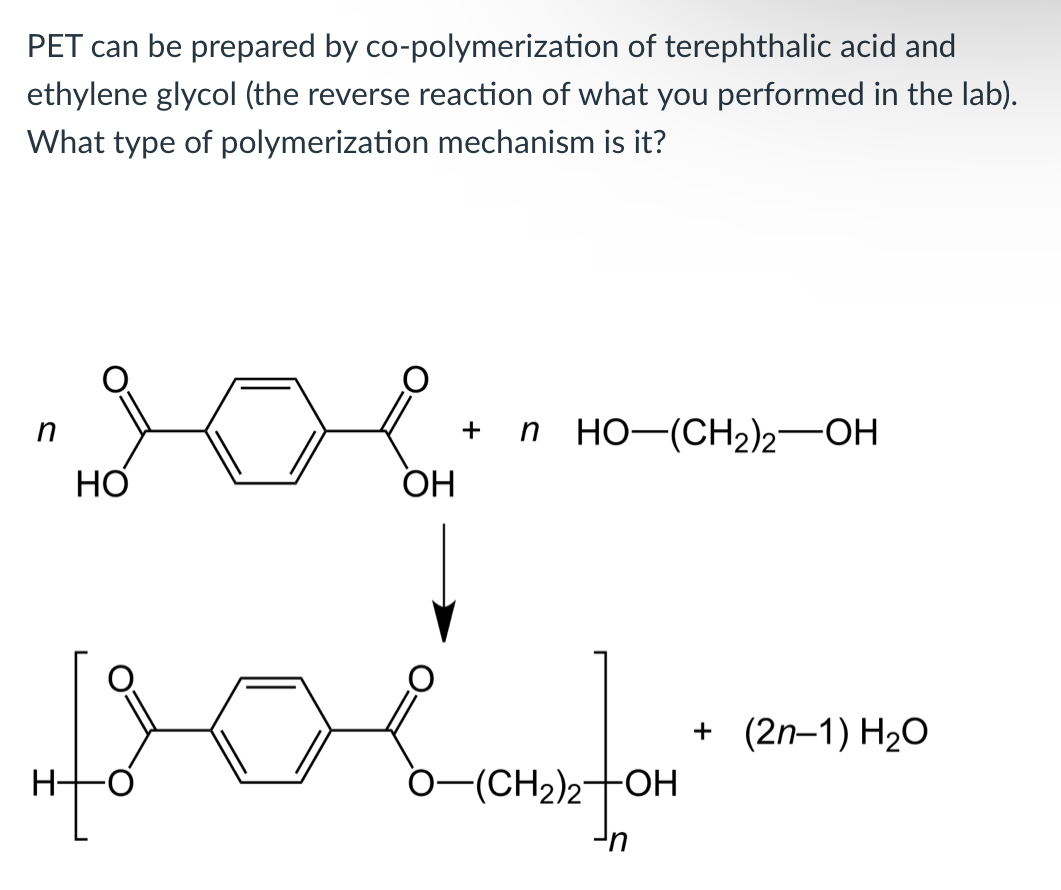
(Lab 8: breaking down plastics)
Condensation polymerization, step-growth
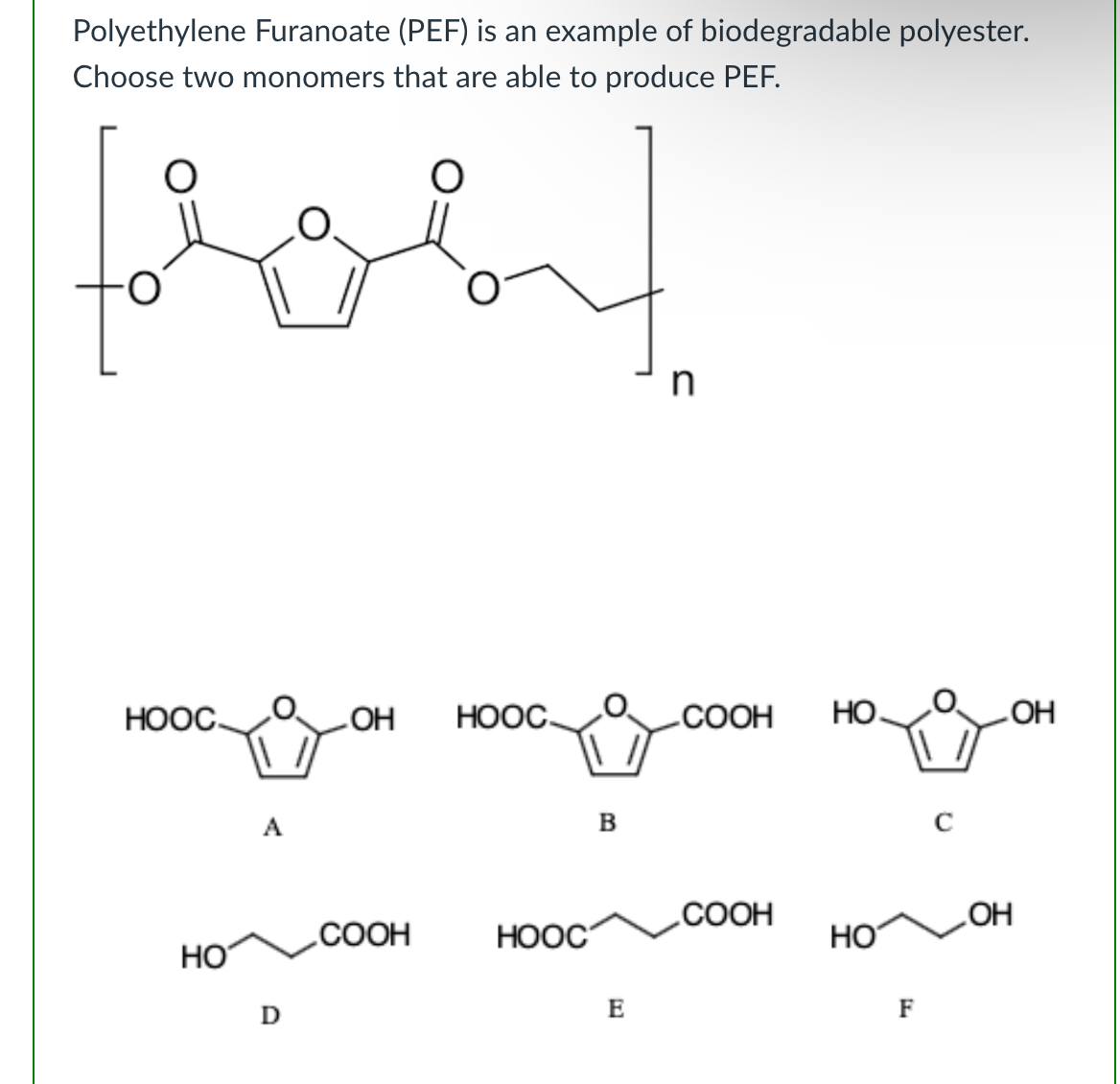
(Lab 8: breaking down plastics)
B and F
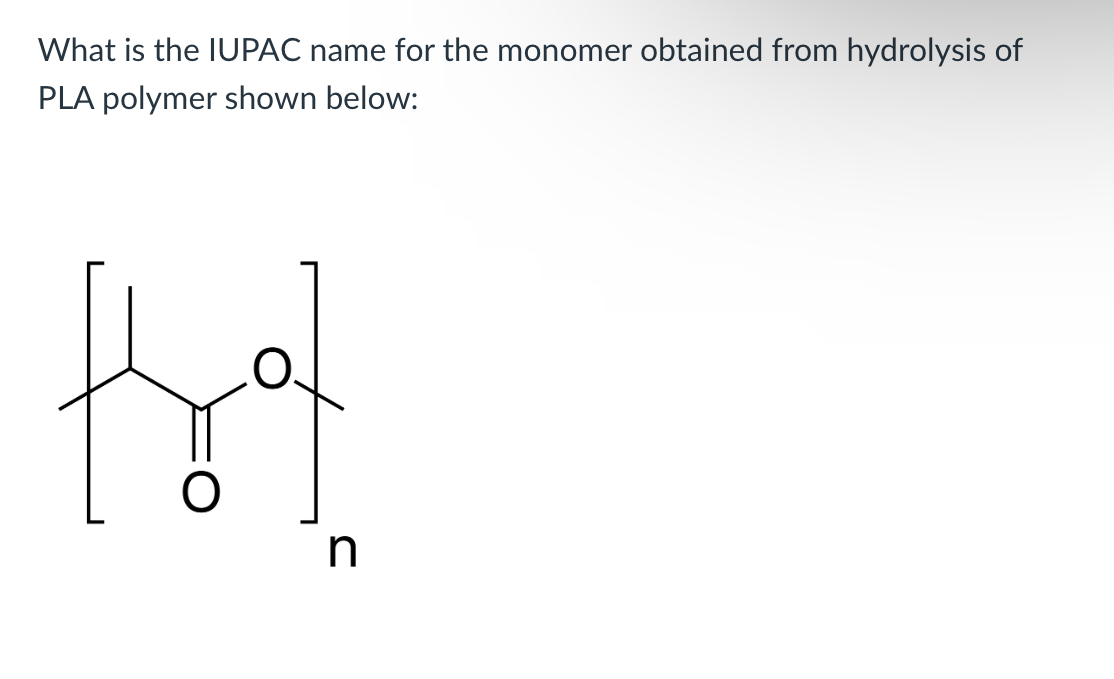
(Lab 8: breaking down plastics)
2-hydroxypropanoic acid
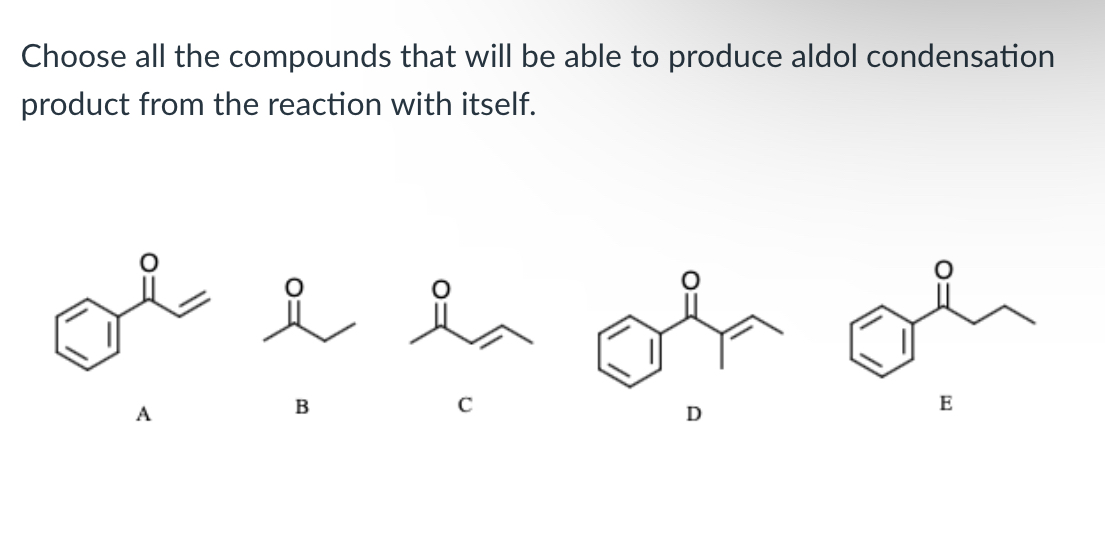
(Lab 9: Aldol )
B, C, and E
What is the main structural feature in the compounds that enables them to participate in the Aldol condensation?
(Lab 9: Aldol )
Presence of a hydrogen atom in alpha position of the C=O group
In Aldol condensation first step that happens is:
(Lab 9: Aldol )
Base deprotonates alpha-position of carbonyl compound
Why does maximum absorption wavelength changes when you switch between the two forms of methyl orange?
(Lab 10: Azo Dyes)
Due the change in conjugated system of methyl orange
In the synthesis of methyl orange, we added NaOH to start the coupling reaction which is best at pH 7 or slightly above
(Lab 10: Azo Dyes)
True
In the mechanism of azo coupling reaction to create methyl orange dimethylaniline acts as a Nucleophile
(Lab 10: Azo Dyes)
True
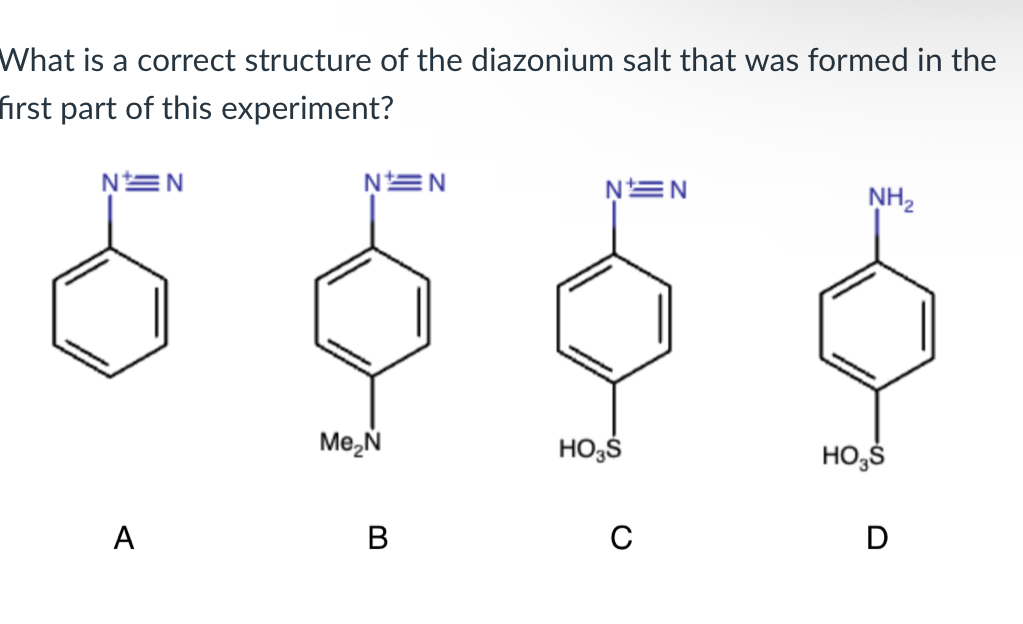
(Lab 10: Azo Dyes)
C
In the textile dying experiment, what type of polymeric material bound to methyl orange the most?
(Lab 10: Azo Dyes)
Polyamide