1.18 - innate immunity 1
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
are innate immunity mechanisms effective in discriminating between host cells and pathogens?
yes
how fast do innate immune defenses kick in?
minutes to hours
fast
when do adaptive immune responses begin?
only when innate defenses are overwhelmbed/bypassed/evaded
describe the differenr barriers of the innate immune system and how they work
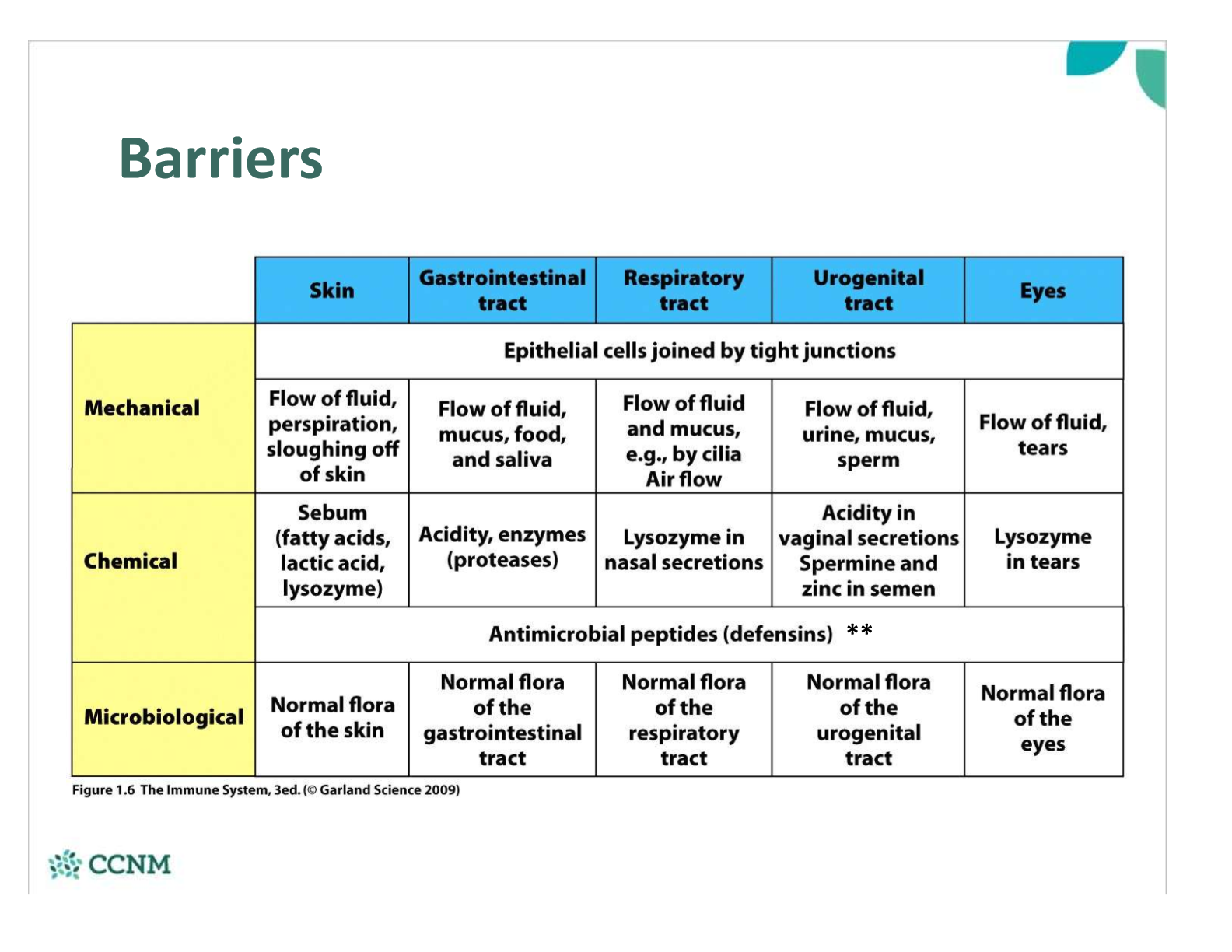
where is Lysozyme located? what does it do?
chemical barrier
Present in secretions (mucus, tears, milk, saliva)
•Uses hydrolysis to break apart the peptidoglycan wall à lysis of bacterial cell wall
what are defensins? what do they do?
chemical barrier
•Small, heterogeneous, cationic peptides
•kill Gram-negative and Gram-positive bacteria, some enveloped viruses, fungi
•Multiple antimicrobial effects:
§Destabilize membranes and Pore formation in bacterial cell walls
§Proteolytic degradation of bacterial proteins
§Inhibit viral binding and entry
§Inhibit virus particle assembly
what is the role of defensins in neutrophil granules?
•Defensins and other AMPs (i.e. cathelicidins) are also stored in neutrophil granules and can be released within tissues in response to inflammation
§Can kill microbes extracellularly à released when neutrophils die during inflammation
§Can kill microbes intracellularly after a cell (i.e. neutrophil) phagocytoses a pathogen
(dual role: •Defensins can act as a chemical barrier when they are secreted by epithelial cells in a variety of mucosal surfaces)
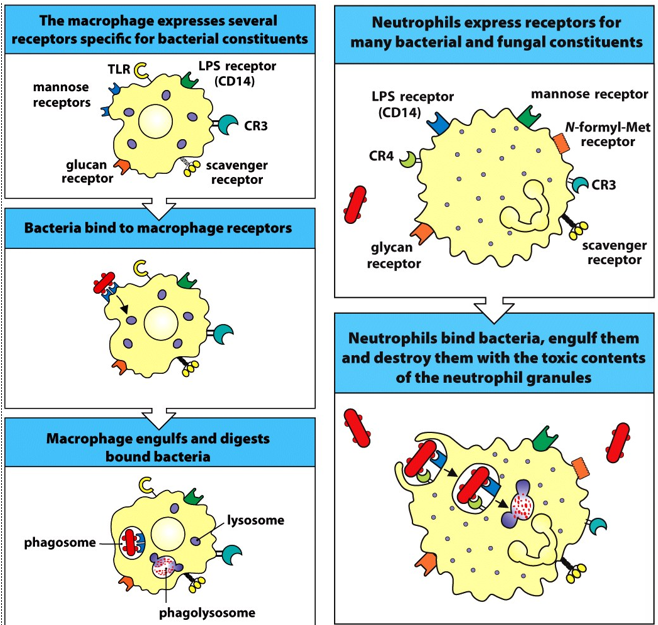
desccribe the imprtance of phagocytosis in immunity
first line of defense
•Key role in innate immunity as they can recognize, ingest and destroy many pathogens without aid of an adaptive immune response
§Phagocytosis can also occur after an antibody has bound to an antigen – the antibody can act as a “signal” that triggers efficient phagocytosis
what are the 2 major phagocytes of the body?
macrophages and neutrophils
describe the lineage, life span and location of monocytes and macrophages
Monocytes & Macrophages:
•Pro-monocytes (BM)àmonocyte (blood)à macrophage/macrophage-like cells (tissues)
•Long-lived cells resident within the tissues
describe the lineage, life span and location of neutrophils
Neutrophils:
•Derived from hematopoietic precursors in the BM
•Non-dividing, short-lived cell type in blood (dominant WBC)
what is a PRR
pattern recognition receptor
describe the process of phagocytosis
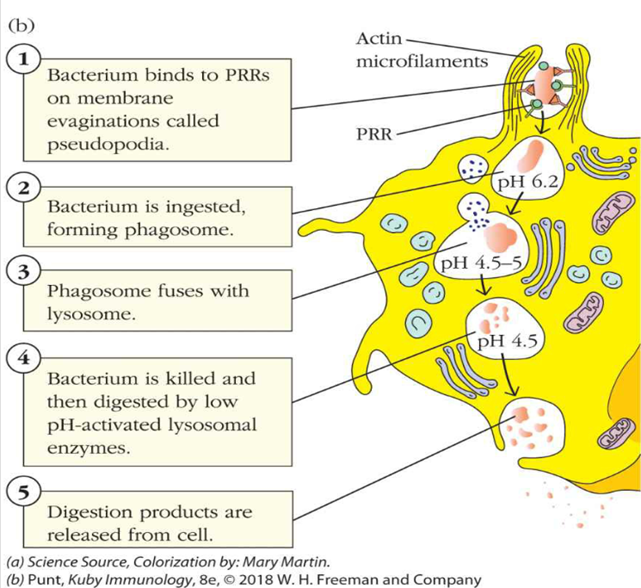
what is pattern recognition
•Evolutionarily conserved mechanism for recognizing common, conserved ‘signs’ of microbial infection, physiological stress, or other damage
how fast is pattern recogniton? does it require prior recogniton? what is the result?
•Recognition is immediate, does not require prior recognition, and activates several arms of the innate (and adaptive) immune response
what do PRRs respond to?
•Responses are elicited via the engagement of Pattern Recognition Receptors (PRRs) found on phagocytes, in response to:
§Pathogen Associated Molecular Patterns (PAMPs)
§Danger Associated Molecular Patterns (DAMPs)
what are examples of PRRs?
§Toll-like receptors
§Nod-like receptors
§Lectins
what are examples of responses elicited by PRRs?
phagocytosis
cytokine secretion
describe step 1 of phagocytosis starting with the PRR
1.A pattern-recognition-receptor (PRR) binds to a microbe or bit of debris, OR an opsonin created by another cell binds to the microbe
•An opsonin is basically a soluble, secreted PRR that enhances the effectiveness of phagocytosis
•An opsonin coats a microbe, the phagocyte has receptors for parts of that opsonin
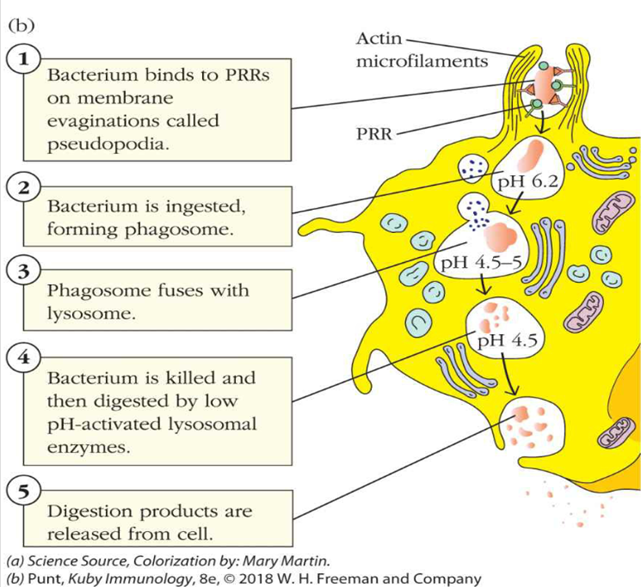
what is an example of a lectin PRR? what does it respond to?

what is an example of a scavenger receptor? what does it respond to?

what fo Fc receptors respond to?

describe step 2 of phagocytosis
2. The microbe is engulfed – the PRR receptors signal the cell membrane to approach, coat and then surround the sites where the receptor is bound
•Forms a phagosome
•Mediated by intracellular signalling events and actin polymerization – see diagram
•PI3 kinase seems to be important here
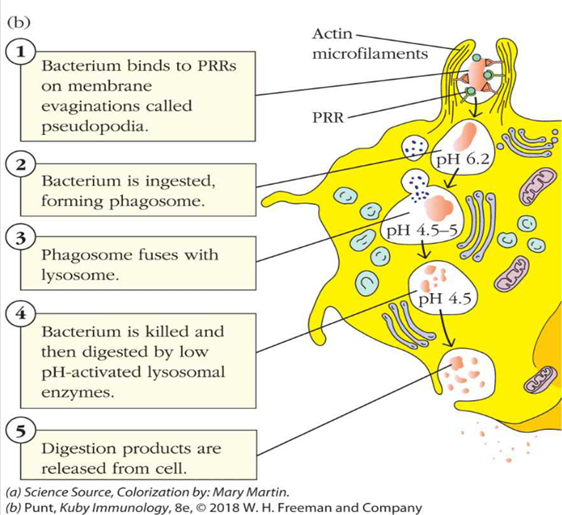
describe steps 3 and 4 of phagocytosis
3 & 4. Microbe killing – phagosomes fuse with lysosomes as well as (in neutrophils) primary and secondary granules
•Phagosomes have many molecules that are effective at cellular killing – a little more later
•Major groups include:
•Reactive oxygen species
•“pore”-forming proteins or peptides
•Hydrolytic enzymes
•pH changes – i.e. acidic environment of the lysozyme
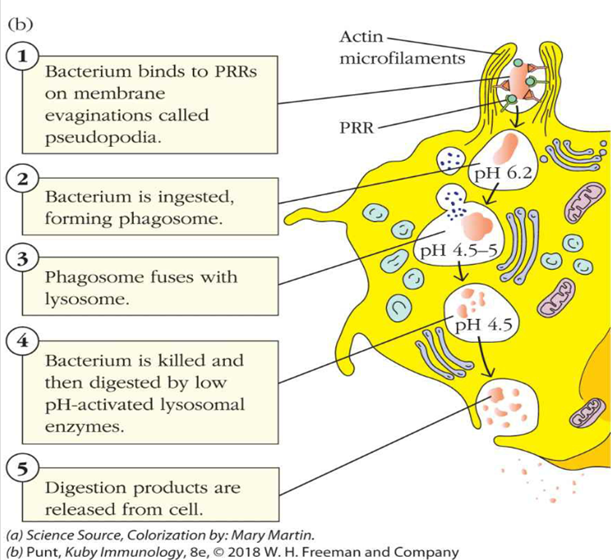
describe step 5 of phagocytosis
5. The microbe remnants are either digested and used, or can be excreted from the phagocyte
describe the possible ways microbes can be killed
lysozyme acid hydrolases/low pH (can oreety much break down anything)
NADPH oxidase complex (becomes assoicated with the membrane of the phagolysosome) causes respiratory burst by providing a lot of Oxygen
macrophages can induce synthesis of high concentrations of nitric oxide to kills
neutrophils have a many pore-forming molecules within their granuales that will fuse with the phagosome
what are the contents of neutrophil granules and what does each do?
Defensins are very rich in cysteine
Form voltage-dependent pores in bacteria that are permeable to water
Cause lysis
Cathepsin - a type of protease
Cathelicidins – another pore-forming molecule
Causes lysis, multitude of different structures
Lysozyme – a glycoside hydrolase
Doesn’t require an acidic pH
Found in a variety of glandular secretions
Great at killing gram +(ve) bacteria
Lactoferrin – iron-binding protein that interferes with iron metabolism in microbes
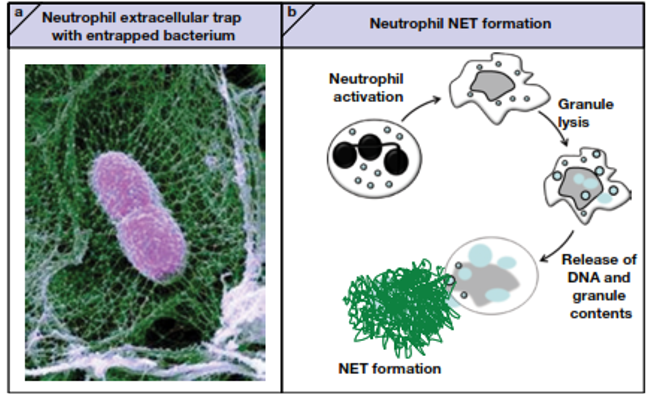
describe NET- neutrophil extracellular trap
•Neutrophils can do a neat trick – when they’re in an environment with many bacteria (they’re “surrounded”) they can lyse and release their DNA into the ECF
§Known as a NET – a neutrophil extracellular trap
§NETs are “sticky” – most bacteria are trapped in the chromatin
§Histones are toxic to many bacteria
§The granule contents will remain close to the NETs and help with killing bacteria, even after the neutrophil itself is dead
describe the family and ligands of Toll-Like Receptors
•Family of 10 cell membrane receptors with variable specificity for a range of pathogens
•Ligands can include LPS, dsRNA, ssRNA, DNA, Flagellin
describe the cytokins released in response to TLRs
Inflammatory cytokines (IL-1b, IL-6, CXCL8, IL-12, TNFa)
Cytokine – a (small) protein messenger, secreted by a vast array of cells, that can:
Influence the differentiation of a wide variety of cells, including leukocytes
Mediate – activate or inactivate – the activity of many cells, including leukocytes
Increase or decrease the production of a wide variety of stem/hematopoietic cells
Interferons
Interferon (IFN) alpha, beta, and lambda (IFNa, IFNb, IFNl)
Autocrine and paracrine signaling molecules that are effective in activating macrophages, NK cells, and inducing an antiviral state
what is MyD88? what happens in people who are deficient in it? in people who always have it turned on?
MyD88 → An essential adaptor in TLR signaling
Patients with MyD88 deficiency:
suffer frequent and severe bacterial infections
Antiviral responses generally unaffected
Patients with constitutively active MyD88:
Develop various blood disorders and blood cancers:
Overproduction or dysregulated production of IgM
B cell lymphoma, marginal cell lymphoma
describe the Nod-Like receptor family and the ligands they respond to
•Family of intracellular receptors found in the cytoplasm that detect products derived from the intracellular degradation of phagocytosed pathogens (e.g. components of bacterial cell wall)
•Also recognize DAMPs associated with cellular stress
•activates expression of inflammatory cytokines
what happens in vasodilation? how does this effect leukocytes?
•Arterioles and pre-capillary sphincters dilate leading to vastly increased blood flow in inflamed tissue
•Vasodilation and fluid loss (due to increased permeability) lead to slower blood flow
§Known as vascular congestion
§This helps with margination of leukocytes
what is the first step of acute inflammation?
A.Alteration of vascular caliber - vasodilation
§Leads to increases in blood flow at the capillary bed due to arteriolar dilation, dilation of precapillary sphincters
•Nitric oxide and histamine
•A variety of prostaglandins (PGI2, PGE2, PGD2)
•Platelet activating factor (at low concentrations – higher concentrations cause vasoconstriction)
•Complement
§C5a and C3a stimulate histamine release
describe the varying effects of nitric oxide depending on its concentration
•At low concentrations, nitric oxide is a potent vasodilator (why Viagra is a profitable drug)
•At high concentrations, it’s capable of destroying both microbes and host cells since it’s a free radical
•Higher concentrations produced by an inducible nitric oxide synthase in macrophages
how are prostaglandins and leukotrienes produced?
•Prostaglandins and leukotrienes are produced when PLA2 generates arachidonic acid from membrane phospholipids
•Different types of cyclooxygenases produce different types of prostaglandins from arachidonic acid
§Important prostaglandins – PGE2, PGD2, and PGI2 cause vasodilation and increase vascular permeability, important acute inflammatory mediators
how are leukotrienes produced?
•Different types of 5-lipoxygenase produce different types of leukotrienes from arachidonic acid that seem particularly important in lung tissue
§LTB4 – important chemotactic agent
§Other LTs – increased vascular permeability and smooth muscle constriction (think asthma)
what is the role of lipoxins in inflammation? how are they produced?
•Lipoxins are generated from arachidonic acid by 12-lipoxygenase – they decrease inflammation
what is the second step of acute inflammation?
B.Enhancement of vascular permeability
§Capillaries and venules become more “leaky” with the release of a number of mediators
•Histamine and serotonin (released by activated platelets, a link between inflammation and clotting)
•Prostaglandins (PGD2 and PGE2)
•Leukotrienes (LTC4, LTD4, LTE4)
•Platelet activating factor
•C3a and C5a
•Bradykinin
§a wide variety of proteins and mediators can enter the interstitial space from the bloodstream
what is trancytosis?
•Active, vesicle-mediated transport across the capillary endothelial cell
•Large molecules can move across the endothelium via:
•Pinocytosis (caveolin pathway)
•Receptor-mediated endocytosis
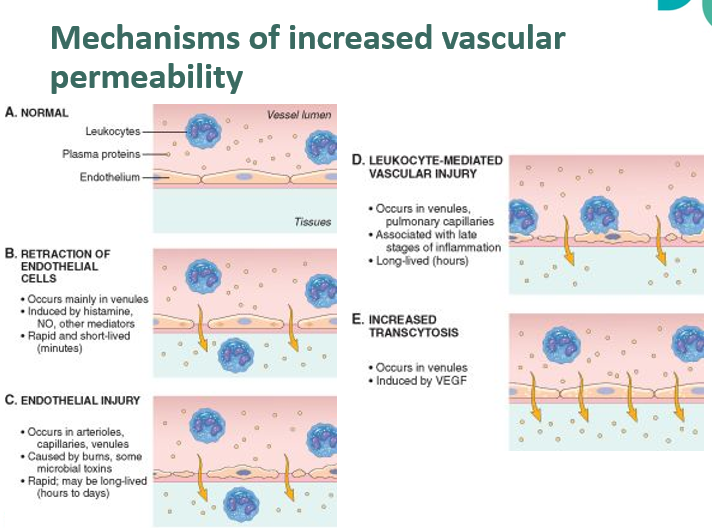
what are the mechanisms that lead to increased vascular permeability?
Increased vascular permeability is due to contraction of endothelial cells
Occurs mainly in venules
Often short-lived
Another mechanism is endothelial damage
Can be caused by trauma, burns, microbial damage
Can also be caused by leukocyte-mediated damage to the endothelium (often longer-lived)
Increased transcytosis can also result in leakage of plasma components into the interstitial space
how is the lymphatic system involved in inflammation?
•As interstitial fluid accumulates during inflammation, pressure increases in the interstitial space and lymphatic drainage increases
§Normally only a small amount of interstitial fluid is produced in non-inflamed tissue
§Excess fluid, microbes, debris, and leukocytes all migrate into the lymph during inflammation
•The lymphatic vessels themselves can become inflamed – known as lymphangiitis
describe leukocyte migration

what is a cytokine? what does it do?
•Cytokine – a (small) protein messenger, secreted by a vast array of cells, that can:
§Influence the differentiation of a wide variety of cells, including leukocytes
§Mediate – activate or inactivate – the activity of many cells, including leukocytes
§Increase or decrease the production of a wide variety of stem/hematopoietic cells
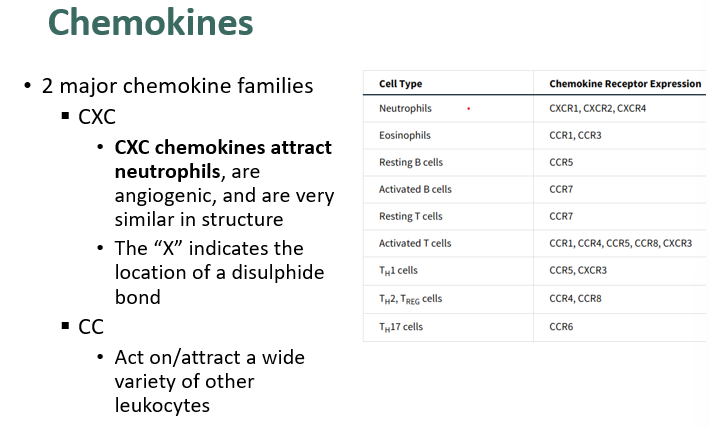
what is a chemokine?
•Chemokine – structurally-related family of small cytokines that:
§Bind to cell-surface receptors (usually leukocytes)
§Induce movement of leukocytes along the chemokine concentration gradient
§Mediate adhesion of leukocytes for the purposes of:
•Differentiation
•Inflammation/migration
what are the 2 mejor chemokine families?
describe leukocyte margination and rolling
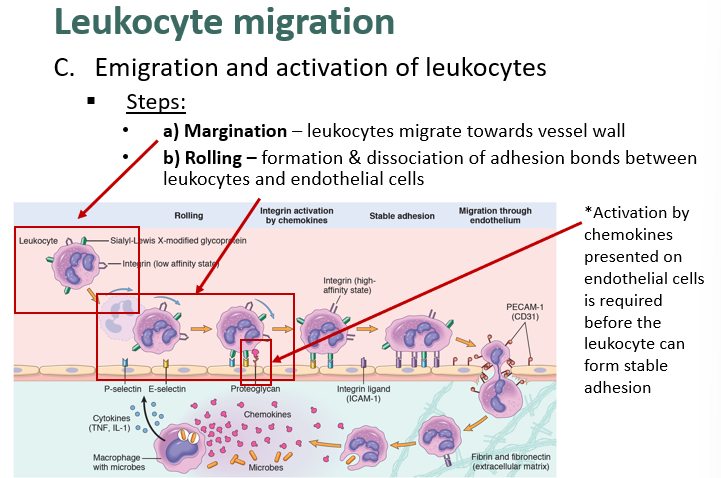
what is the role of endothelial cell in leukocyte activation?
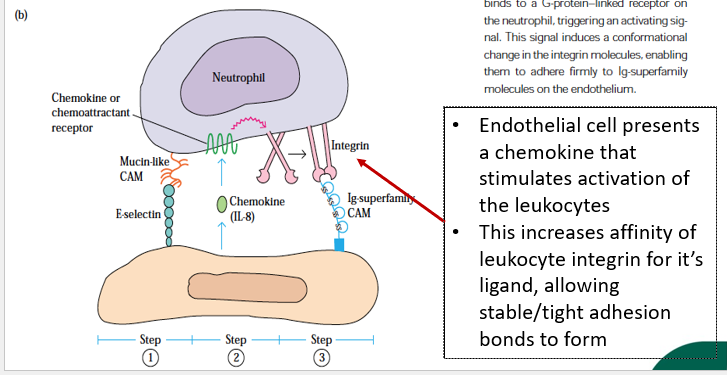
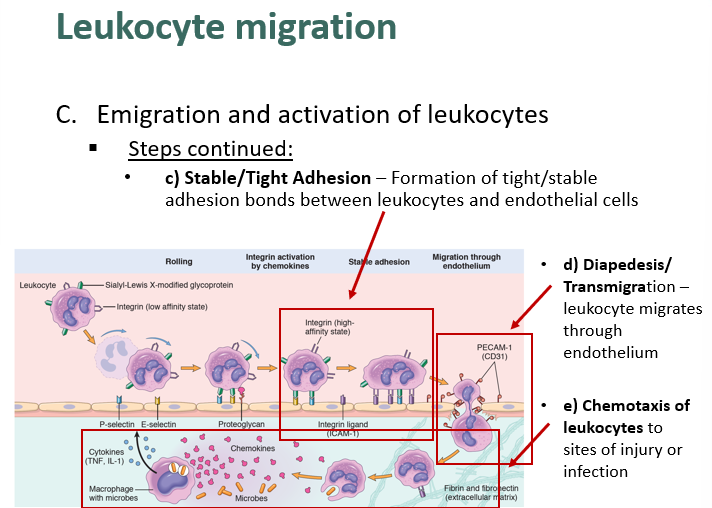
describe stable/tight adhesion, diapedesis and chemotaxis in leukocyte migration
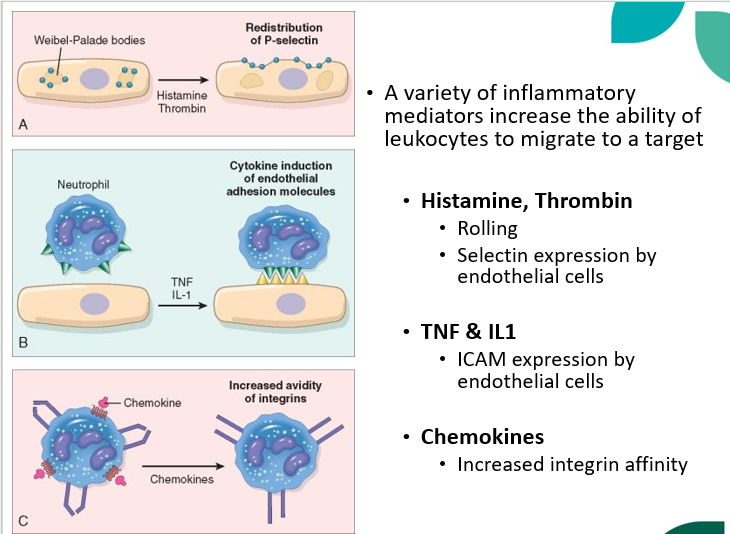
which inflammatory mediators increase the ability of leukocytes to migrate to a target?
what are the chemotactic agents? where are they produced? what do they do?