Chemistry - Unit 4 - The Periodic Table
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Groups:
-The columns of the periodic table are called "groups" and are numbered 1-18
-Elements in the same group share similar characteristics
-All elements in a group have the same number of valence electrons
-Similar chemistry
-When you compare elements in the same group, the number of energy levels increases as you go down
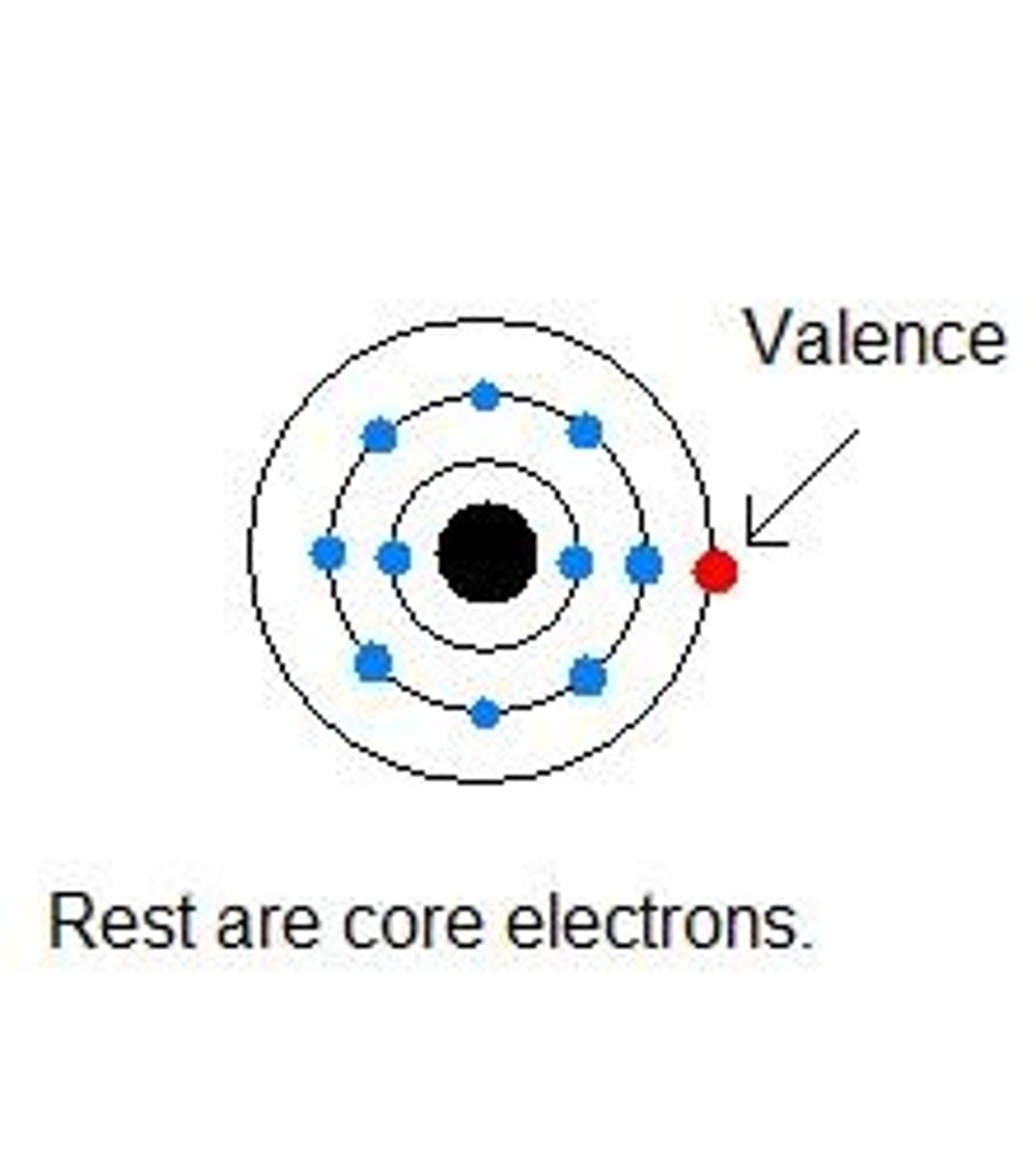
Valence Electrons
-Determine what kind of chemical reaction an elements does or doesn't
-The valence electrons are the outermost electrons in an atom, meaning it is the one that interacts with other valence electrons
Periods
-Rows on the periodic table are called periods
-All elements in a period have the same number of energy levels
-When comparing elements in the same row, elements have the same number of energy levels but different number of protons. Protons in the nucleus increases
-same number of energy shells
-least at top, most at bottom
-different numbers of valence electrons, different chemical reactivity
Mendeleev's Original Periodic Table
-Organized by atomic weight
-Predicted other elements and their properties
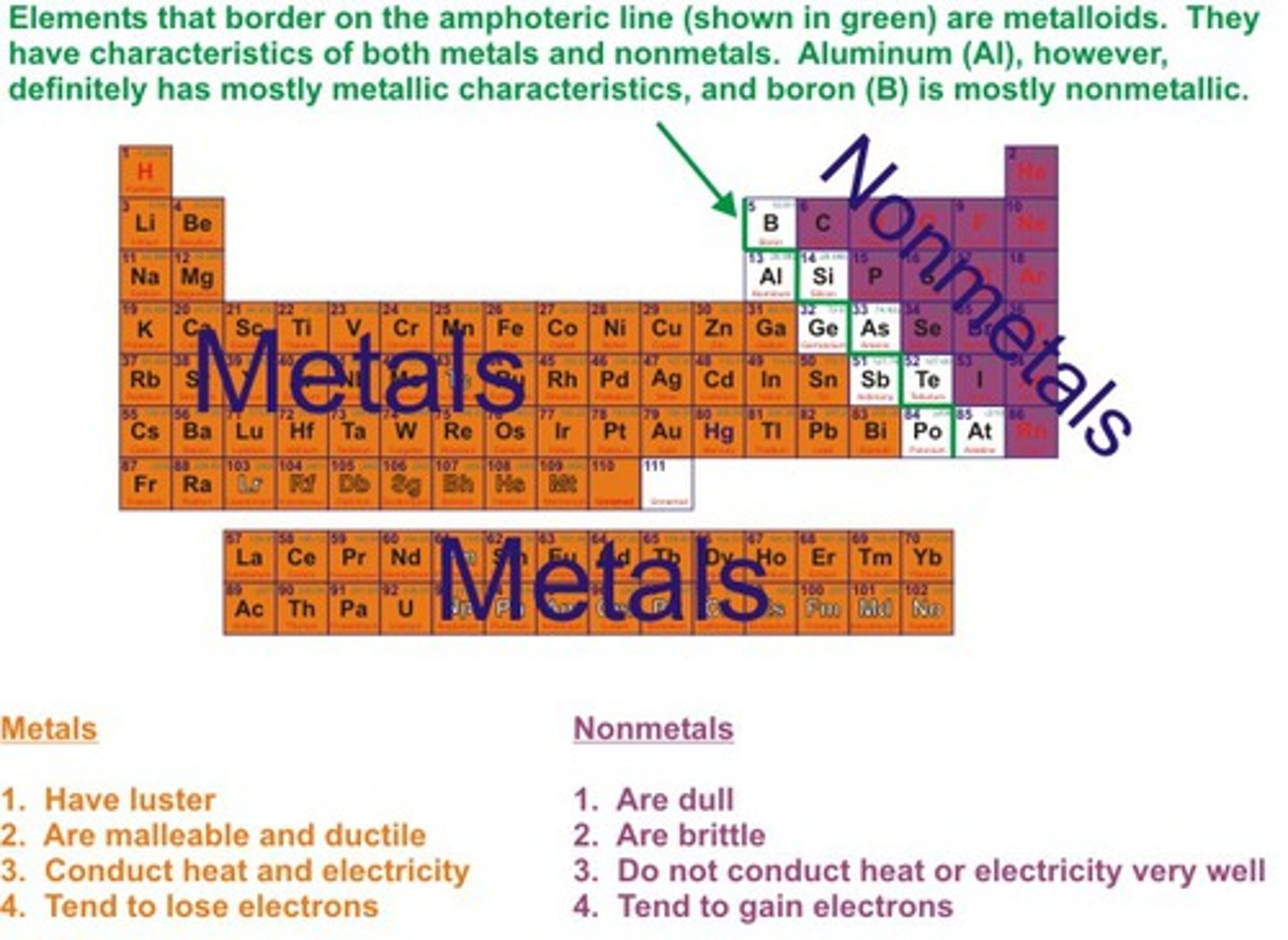
More metals or nonmetals in the periodic table? And where are the metals located on the periodic table?
-Most of the elements on the periodic table are metal
-Group 0-12 (excluding H) and Al, Ga, In, Ti, Sn, Pb, Bi, Po)
Attraction between particles (what do we pay attention to)
-When in the same group/column we pay close attention to the energy levels
-When in the same period/rows we pay close attention to number of protons
Metals:
-Good conductors of heat and electricity
-High luster (shiny)
-Malleable (can be made into thin sheets)
-Ductile (can be made into thin wires)
-Mostly solid at STP (standard temperature and pressure)
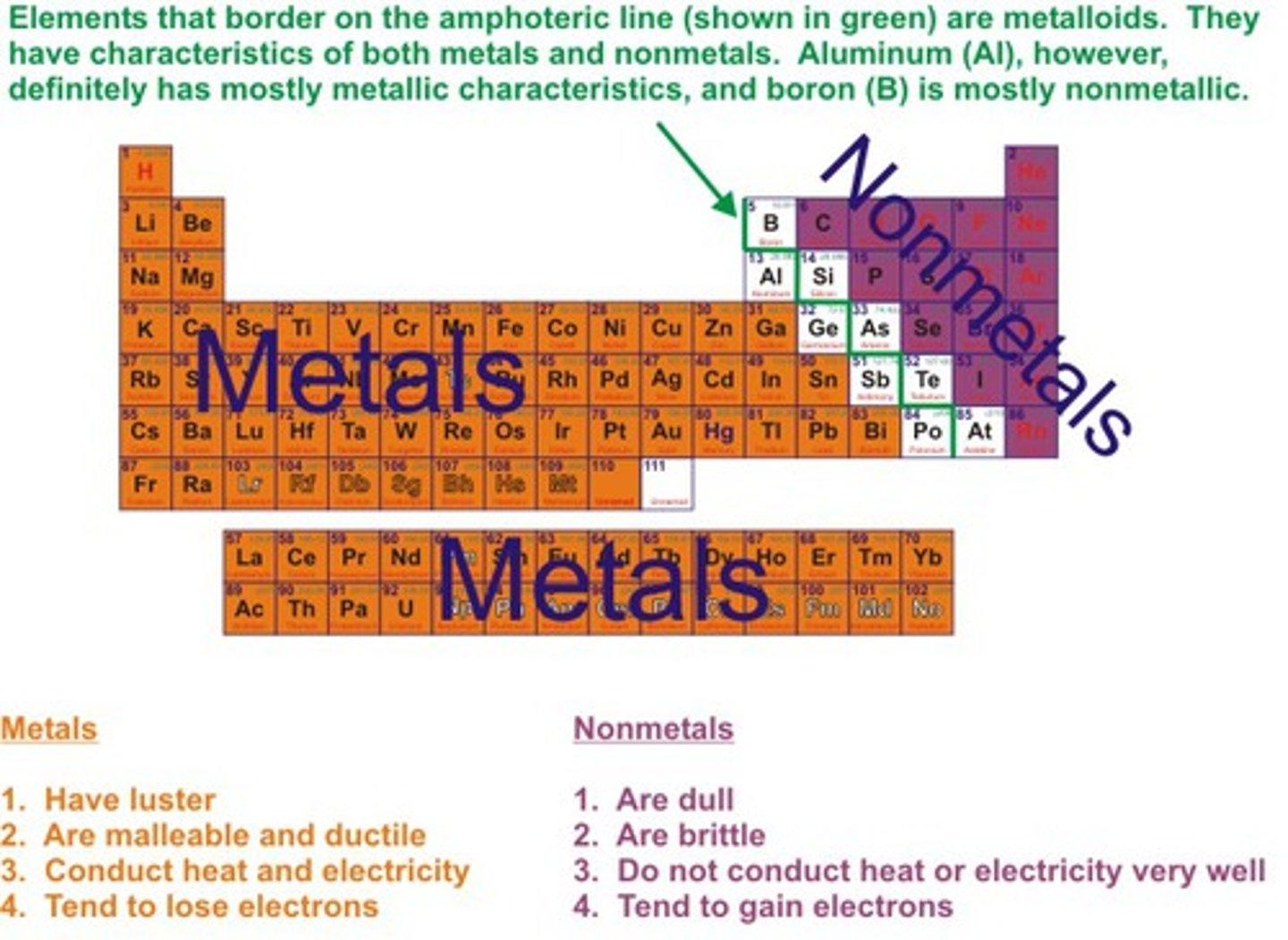
Nonmetals:
-Not good conductors
-Not malleable
-Not ductile
-Not shiny (dull)
-Are often brittle
-opposite of metals
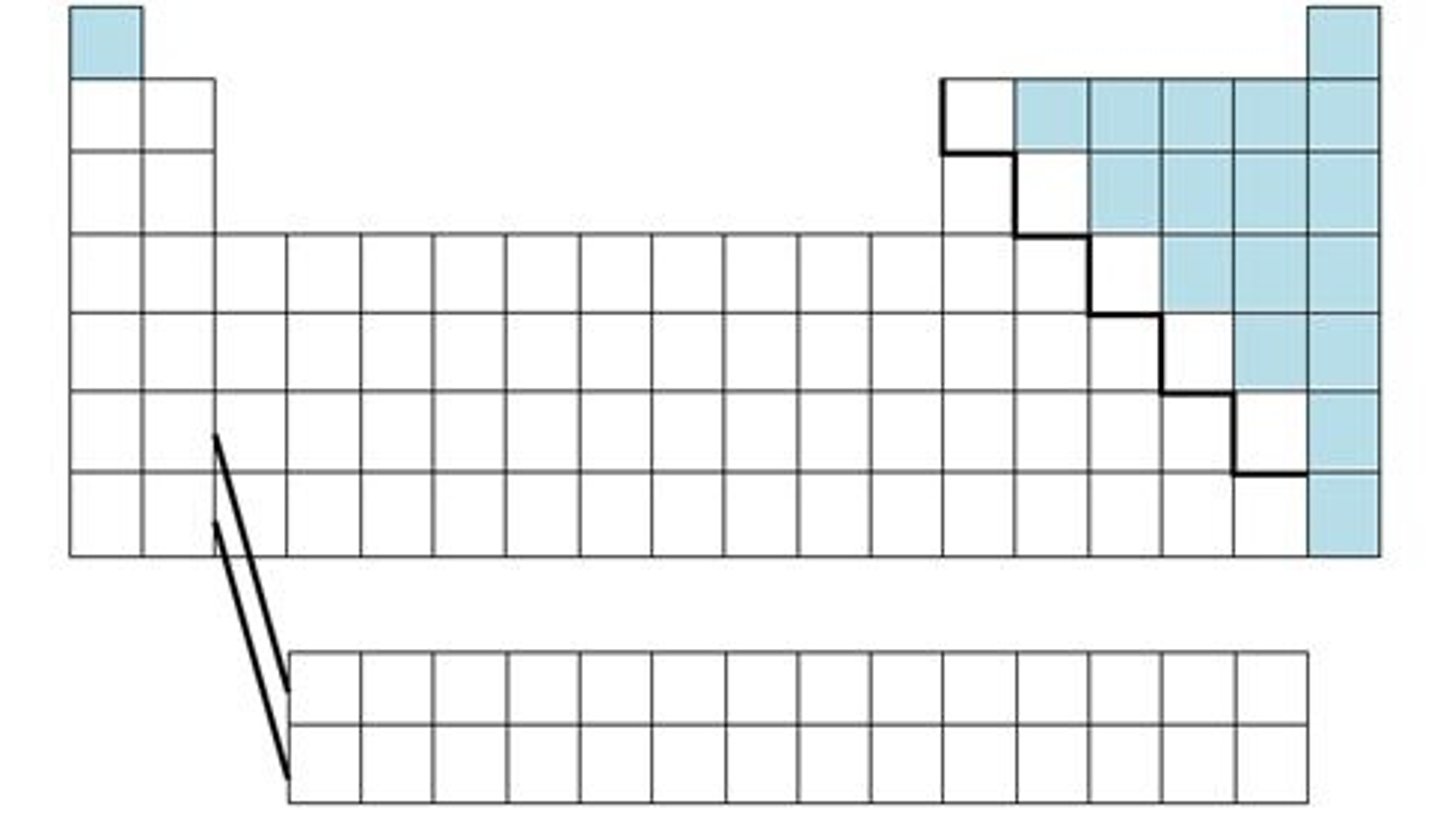
Metalloids:
-Mix of properties of metals and nonmetals
-Found along the thick steeped line (Be, Si, Ge, As, Sb, Te, At)
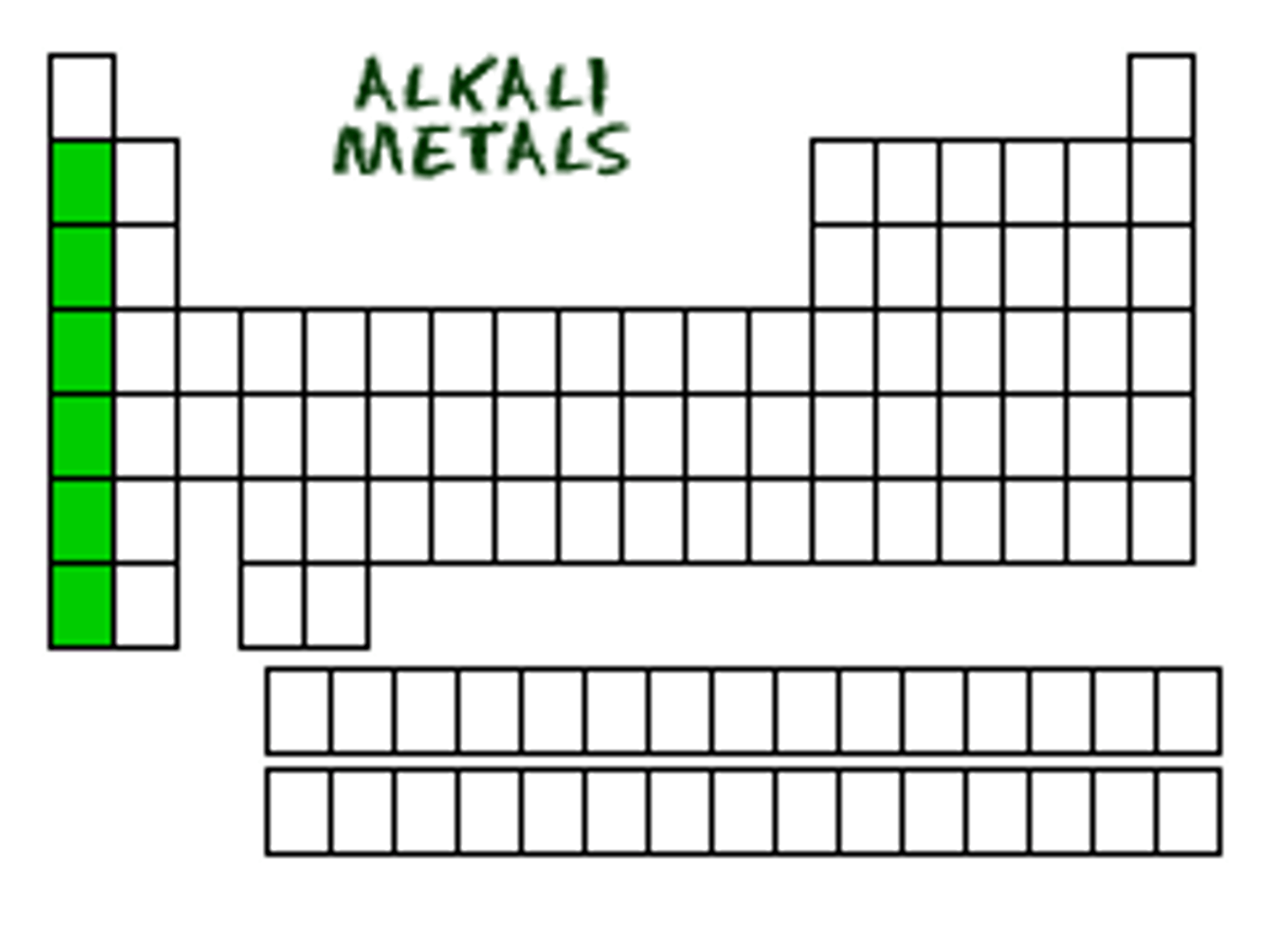
Group 1
(ve, reactive or no, exist by themselves in nature?
-1 valence electron
-very reactive
-don't exist by themselves in nature
ALKALI METALS
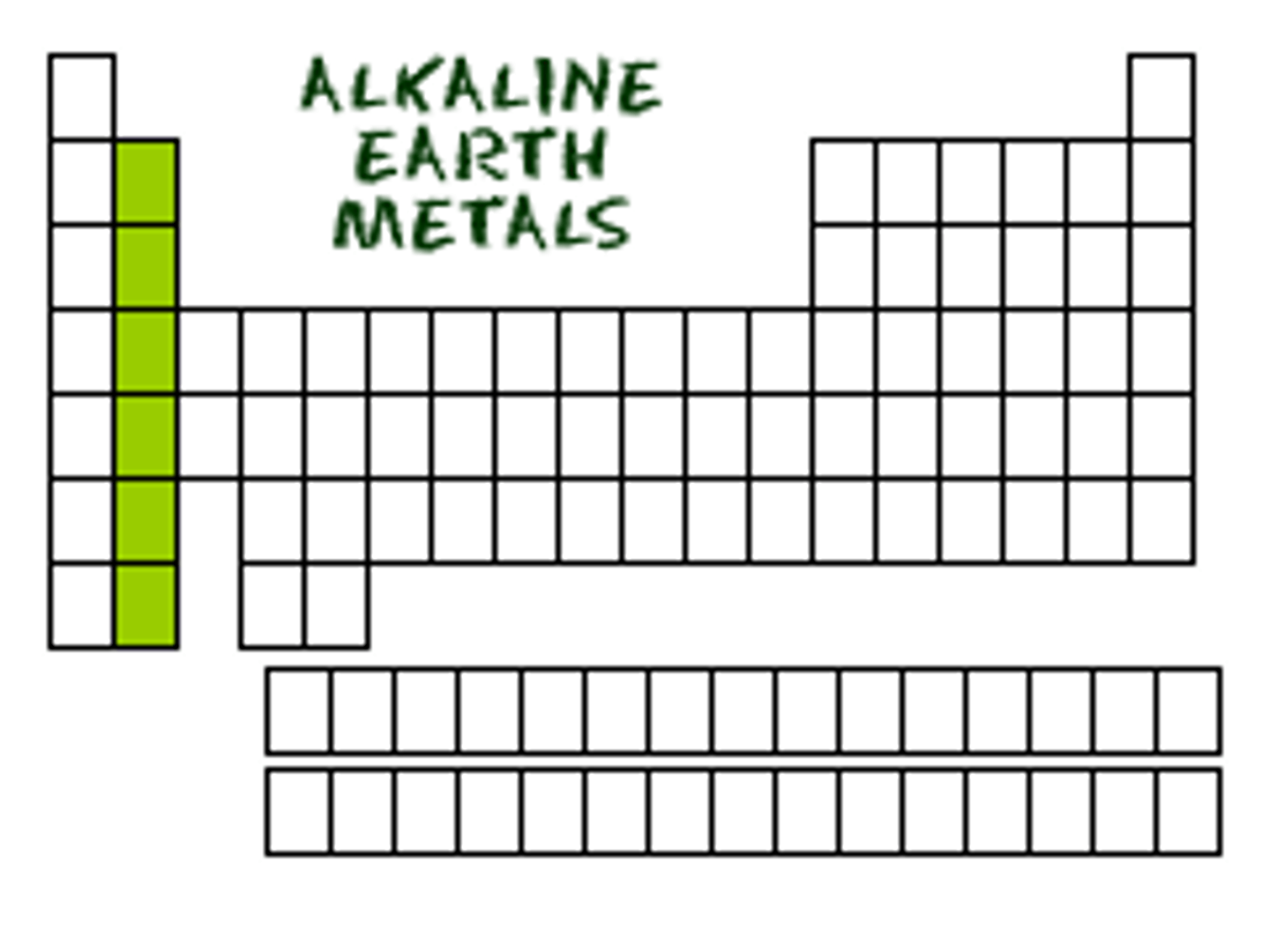
Group 2
-2 valence electrons
-very reactive but not as much as group 1
ALKALI EARTH METALS
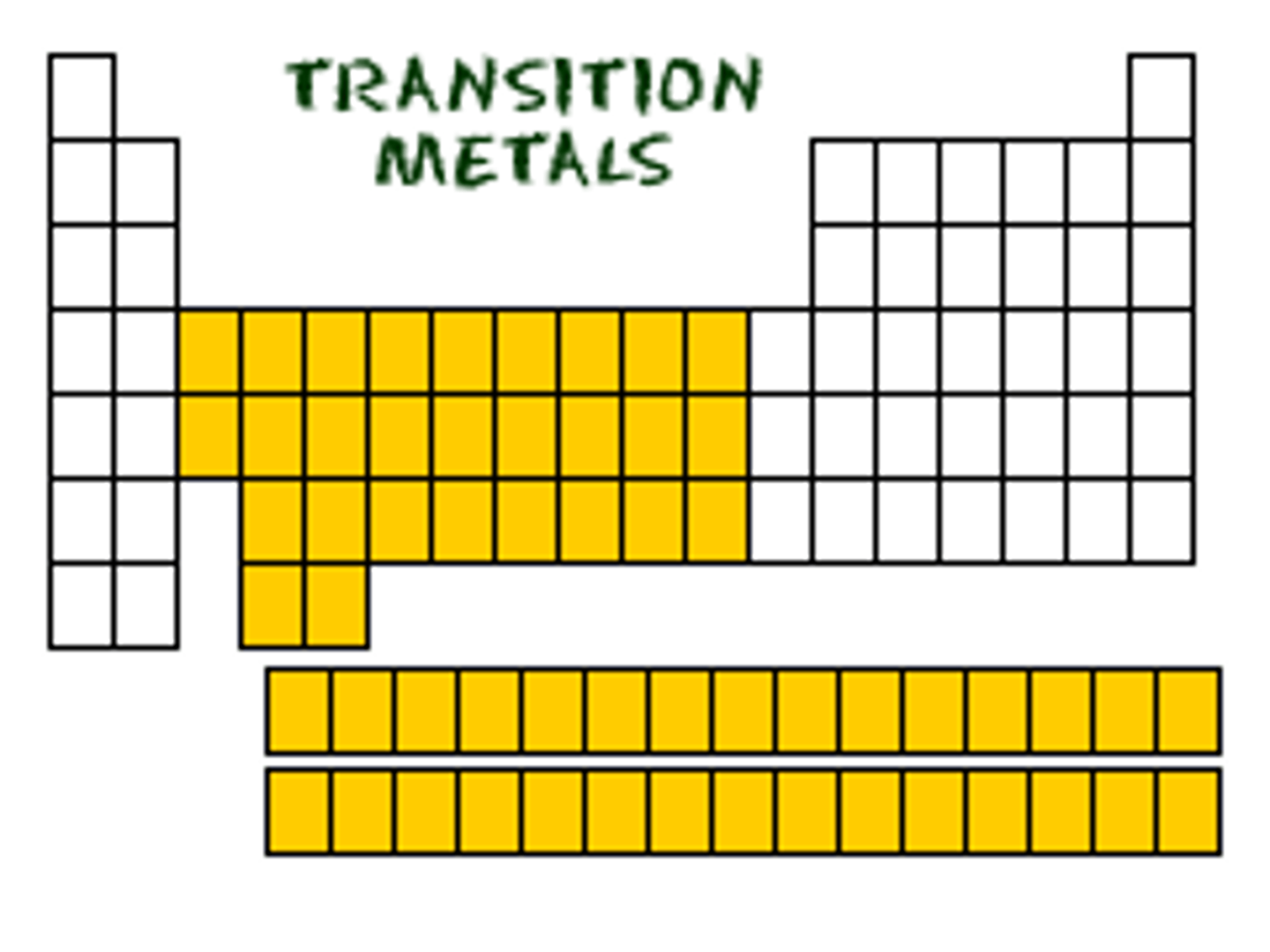
Group 3-12
-Multiple forms
-Multiple possible valence electrons
-Create colorful aqueous liquids
-more than one oxidation state
TRANSITION METALS
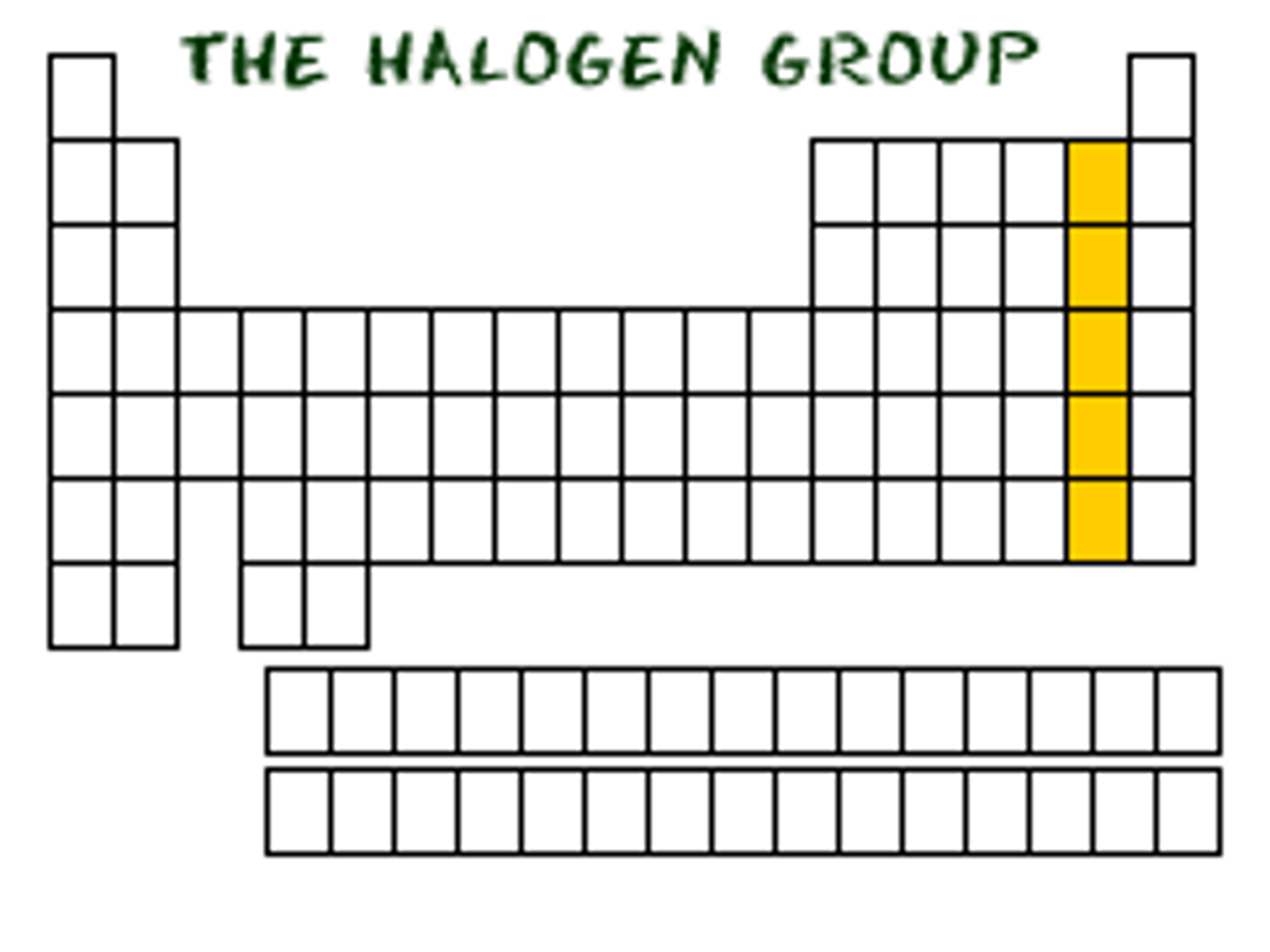
Group 17
-7 valence electrons
-Very reactive and high electro negativity and ionization because they are very close to having a full set of valence electrons
-Exist in nature as pairs (diatomic)
HALOGENS
Diatomic
-Molecules composed of 2 atoms

Group 18
-8 valence electrons (except for Helium (2))
-Not reactive (inert)
-VERY STABLE
Exist in nature by themselves (monatomic)
-Full valence electrons
-low electronegativity and ionization because they don't want to change
-OTHER ELEMENTS WANT TO BE STABLE AND UNCHANGING LIKE NOBLE GASSES WHICH IS WHY THEY GAIN/LOSE ELECTRONS
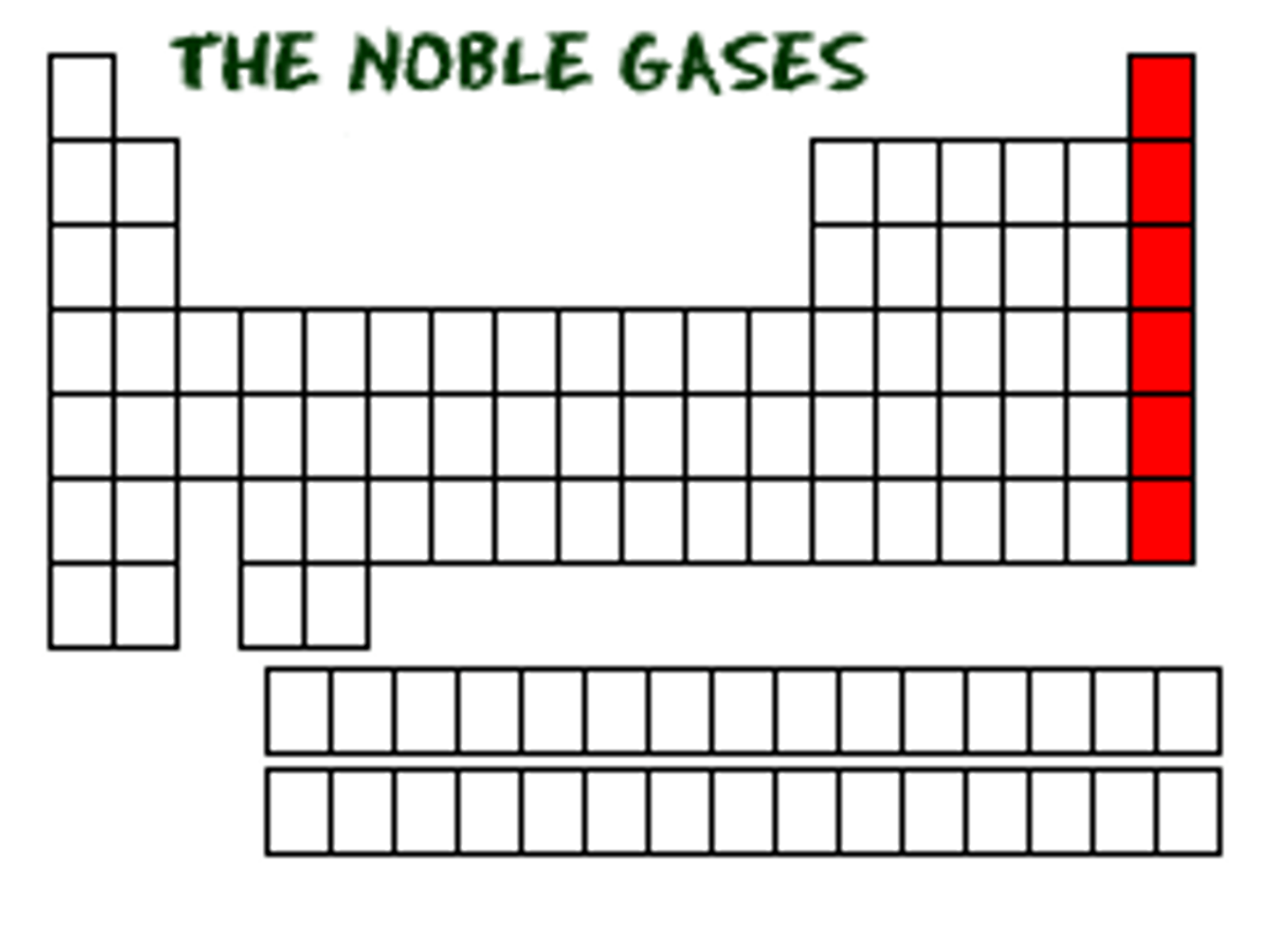
NOBLE GASES
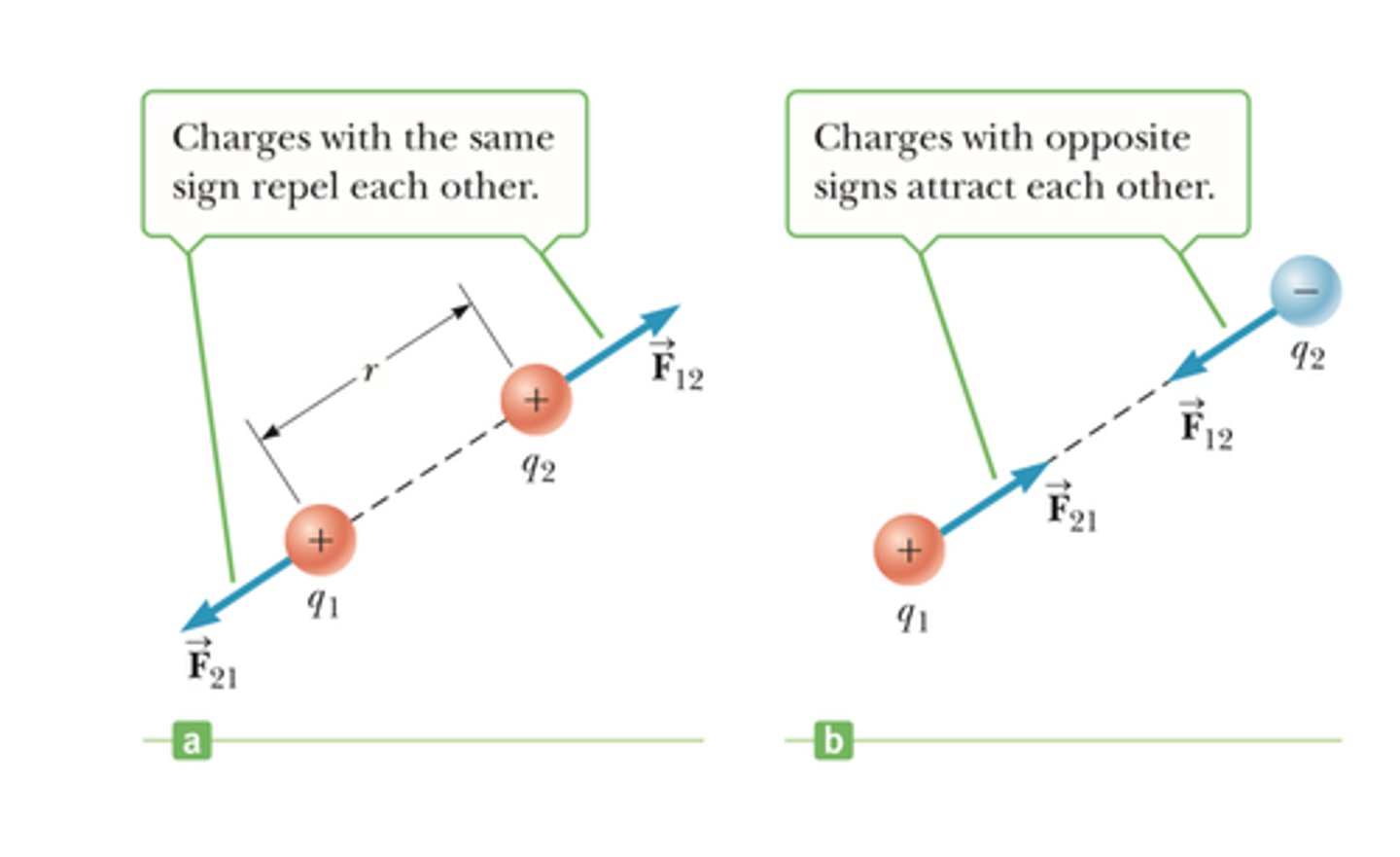
Coulombic attraction:
-The attraction between oppositely charged particles
-Can be weak or strong
-between protons and valence electrons
Do other elements want to be like Noble Gasses?
Yes!
-Other elements want to be stable and unchanging like noble gasses, which is why they gain/lose electrons
-It is easier and requires less energy to stay the same then to constantly be changing
What is the pattern in nature?
-Atoms will gain or lose electrons in order to have 8 valence electrons.
-Hydrogen and Helium are the exception, both only have one energy level so can only have a max of 2 valence electrons.
- If atoms are close to 8 valence electrons they will strongly attract other electrons (electronegativity) and do not want to lose valence electrons (high ionization energy)
-If atoms are not close 8 valence electrons they will not strongly attract other electrons and will be more likely to lose valence electrons (low IE) -Cations, positive (pawsitive), lose electrons
-Anions, negative, gain electrons
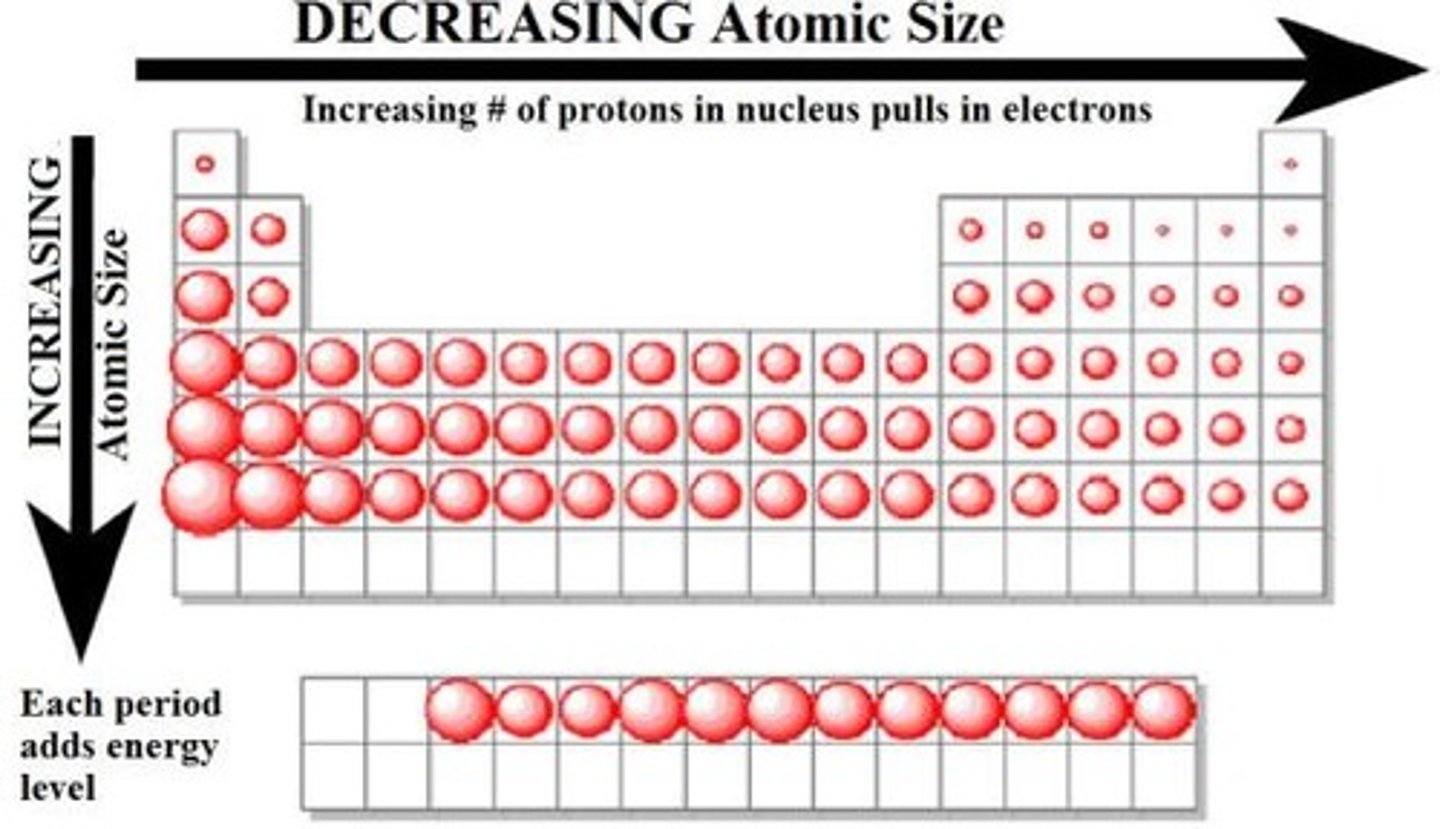
Atomic Size:
-Decreases as you go up and to the right on the periodic table
-As you go up there are less energy levels and as you go right there are more protons pulling on the valence electrons so they are closer to the nucleus
-more electronegativity = more pull so less space between nucleus and valence electrons so smaller atomic radius
-distance from nucleus to valence shell
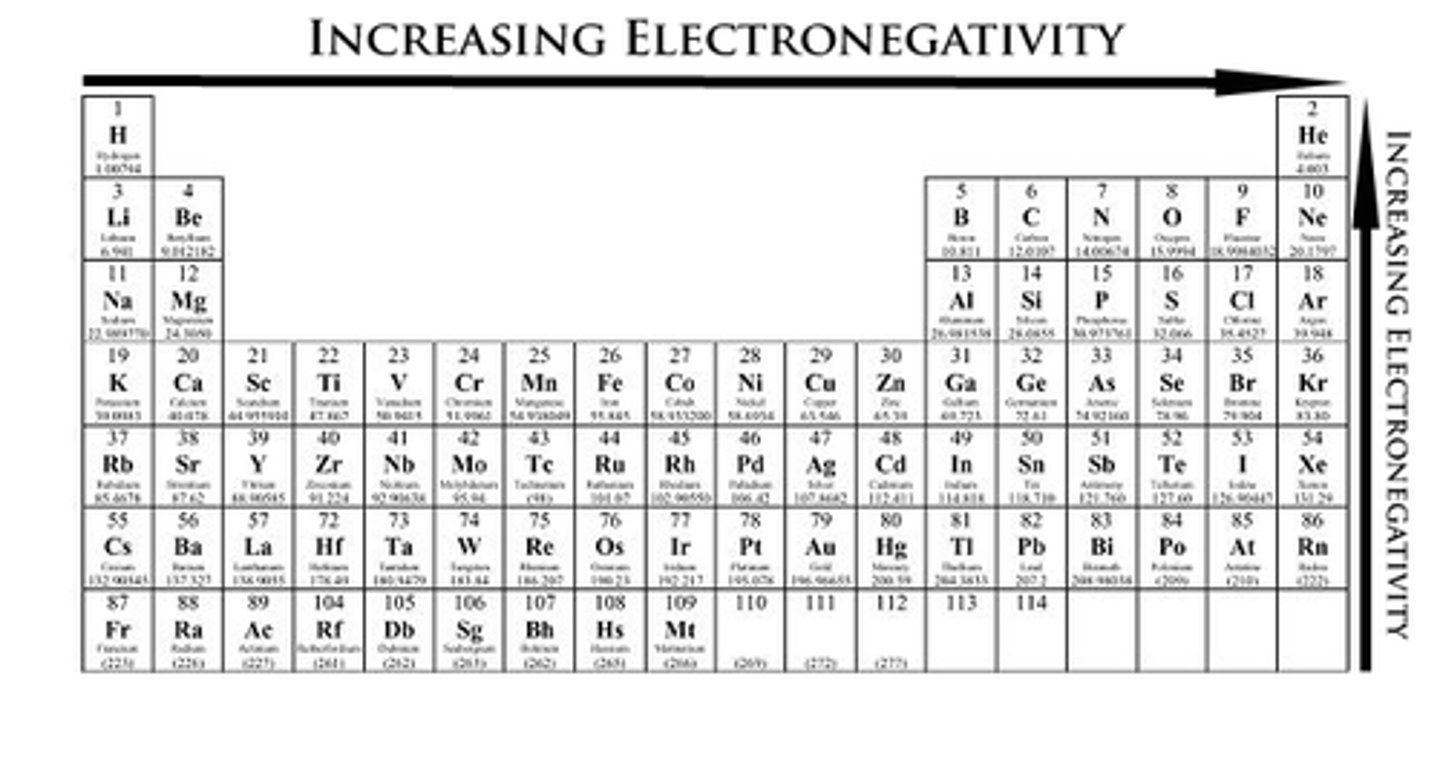
Electronegativity:
-Increase as you go up and to the right on the periodic table
-Smaller atoms are generally more electronegative (participate more in bonding)-As you move to the right atoms tend to attract electrons (to be like noble gasses)
-tendency for an atom to attract shared electrons when forming a chemical bond
Ionization Energy:
-Increases as you move up and to the right on the periodic table
-Smaller and more electronegative atoms (stronger attraction between nucleus and valence electrons) are harder to take valence electrons from, so ionization energy increases
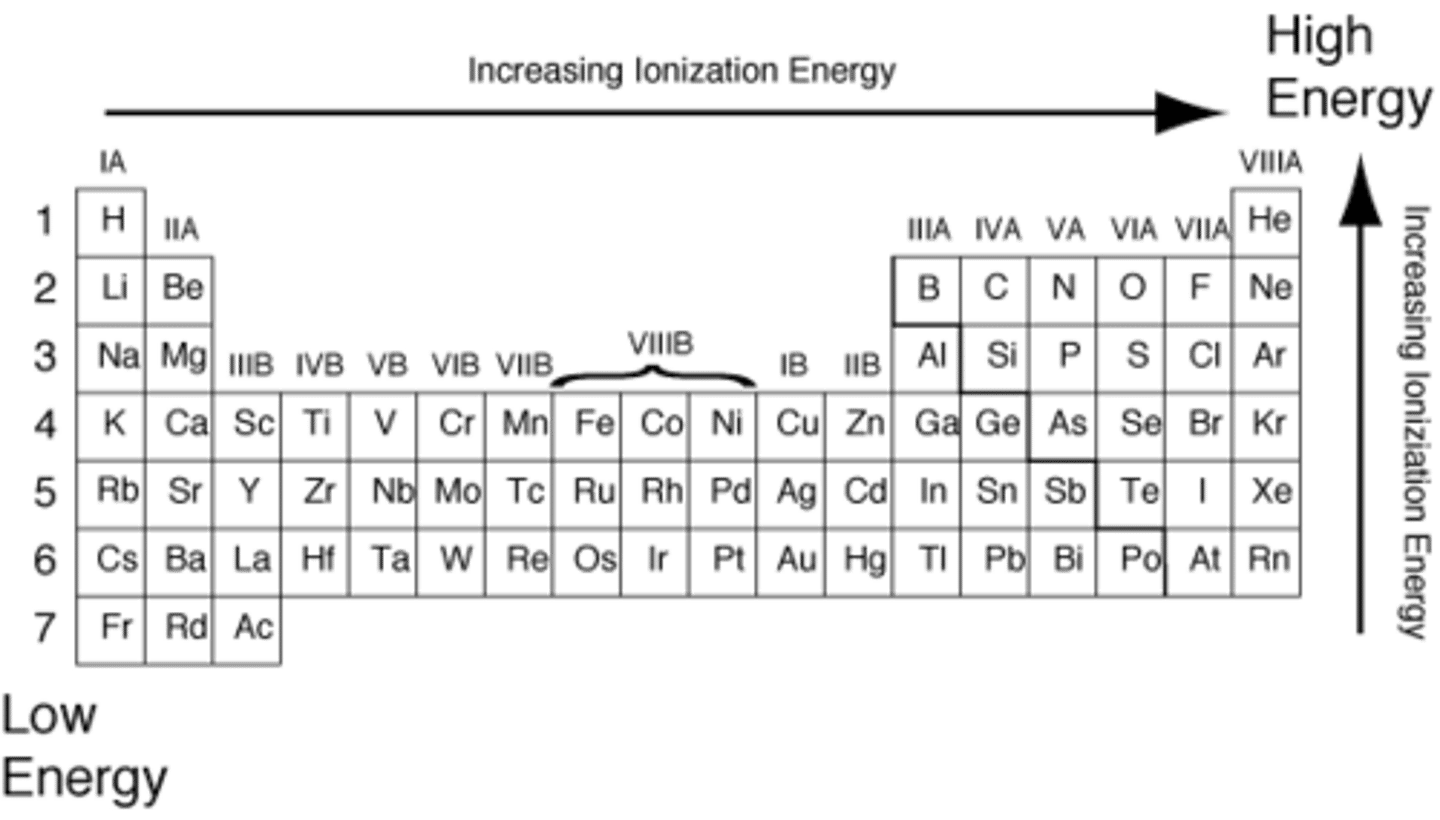
The atoms that are naturally stable:
-The atoms that are naturally stable don't want to gain or lose electrons and exist by themselves in nature are the Noble Gases (group 18)
-Other elements will gain or lose electrons to have the same electron configuration as the noble gasses
Ionic Size:
-Adding electrons to an atomic increases its size
-Removing electrons reduces its size
-If you are comparing ions/atoms with the same amount of valence electrons, the one that has the most protons in the nucleus will be the smallest
-Adding or removing electrons changes the charge, charge is also called oxidation state
-NO overall trend for ionic size
-Depends on the amount of electrons an ion gained or lost and how many protons are in the nucleus
Reactivity
-the ability of a substance to interact chemically with a second substance
-The number of electrons in the outermost shell of an atom determines its reactivity.
- Noble gases have low reactivity because they have full electron shells.
Name all the group names in order, what groups and what name. Also give electronegativity, atomic radius, and ionization energy (low, high, and why) as well as valence electrons
Group 1 - Alkali Metals, 1 ve, very reactive
Group 2- Alkali Earth Metals, 2 ve, attractive but not as much as group 1
Group 3-12 - Transition Metals, MULTIPLE forms, valence electrons
Group 17- Halogens, 7 ve, very reactive because they are super close to having a pull valence electrons shell
Group 18, Noble Gases, 8 ve (full shell), inert (not reactive) low ionization and electronegativity because they don't want to change
Octet Rule
States that atoms lose, gain or share electrons in order to acquire a full set of eight valence electrons
What determines if two elements share similar chemical properties?
If they are in the same group. AKA have the name number of valence electrons.
When a positive ion (cation) is formed the radius ——-
Is smaller than a normal atom because it loses electrons
How are isotopes written and how can you tell two isotopes of the same element?
-atomic mass on top, atomic number on bottom
-same atomic number, different atomic mass
What's the biggest difference between the old periodic table and the new periodic table?
Atomic mass organization for OG
Atomic number organization for Modern one
Atomic mass
The weighted average of naturally occurring isotopes of a certain element
Orbital
High probability of finding an electron
What determines if an atom is more likely to form an anion or a cation?
First oxidation state