Haemoglobin
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is haemoglobin and describe its structure.
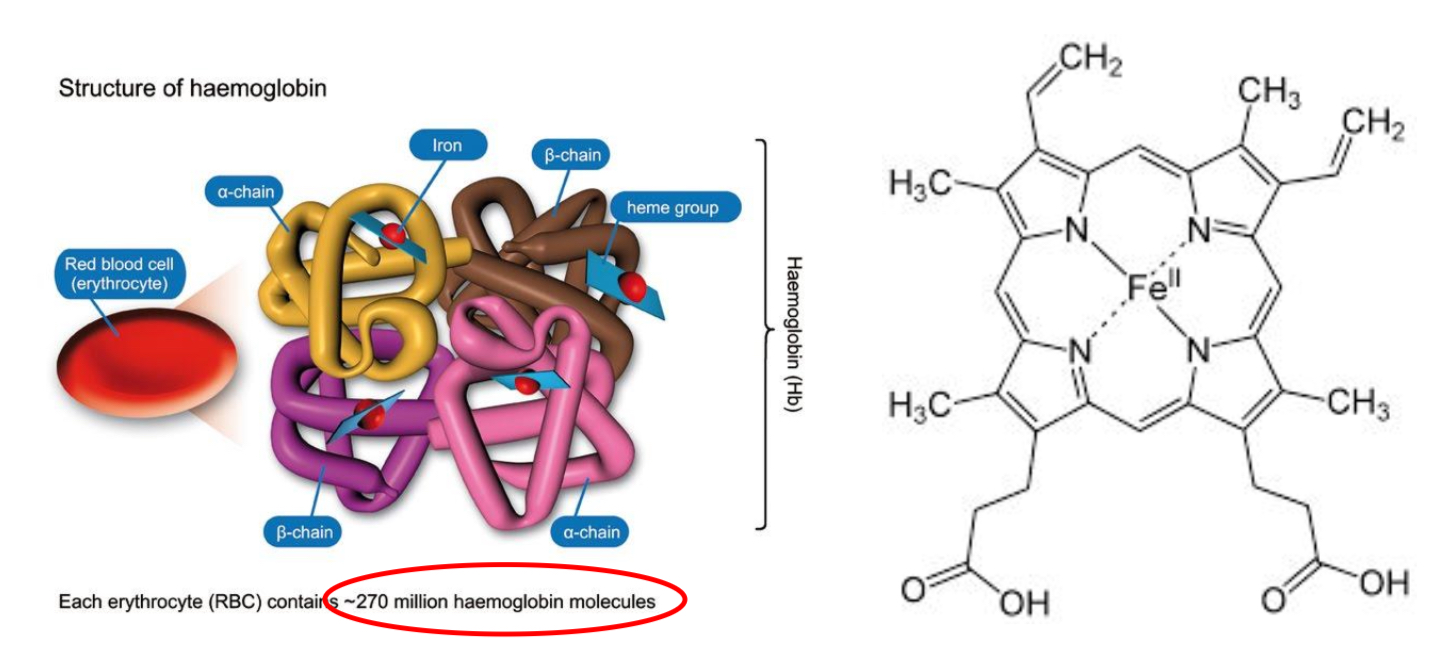
Groups of proteins with a quaternary structure found in different organisms and transports oxygen.
Has four polypeptide chains which each contain a heme group and iron where the oxygen binds to.
Define affinity of haemoglobin for oxygen.
The ability of haemoglobin to attract, or bind to oxygen.
Define saturation of haemoglobin with oxygen.
When haemoglobin is holding the maximum amount of oxygen it can bind.
Define loading/association of haemoglobin.
The binding of oxygen to haemoglobin.
Define unloading/dissociation of haemoglobin.
When oxygen detaches or unbinds from haemoglobin.
What can the shape of an oxygen dissociation curve be described as?
A sigmoid curve
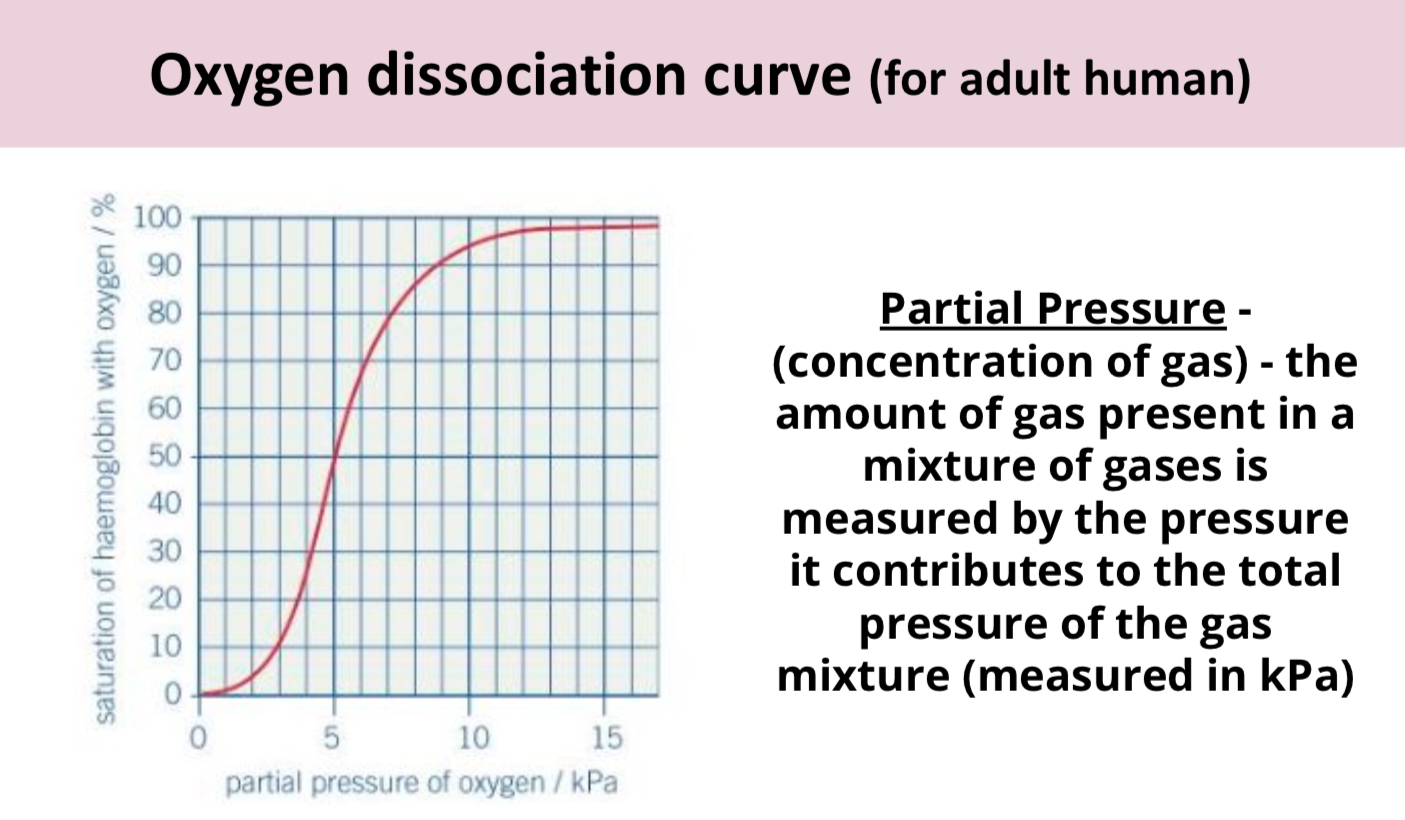
Describe what’s happening at lower and higher partial pressure in this oxygen dissociation curve.
At lower partial pressure of oxygen (e.g. in respiring cells) haemoglobin has quite low affinity meaning its likely to unload oxygen (good because respiring cells need more oxygen).
At high partial pressure of oxygen (e.g. in alveoli) haemoglobin is almost completely saturated with oxygen meaning it has loaded more oxygen (good because oxygen needs to be loaded to be transported to respiring cells).
Define cooperative binding and how that links to haemoglobin.
It means the binding of one molecule affects the binding affinity of subsequent molecules.
Haemoglobin and oxygen have a cooperative nature as haemoglobin changes shape when the first oxygen binds. This then makes it easier for further oxygens to bind. That’s why we have a steep curve since it’s easier for oxygen to bind.
Define the Bohr effect.
Bohr effect links to three factors: high carbon dioxide concentration, low pH and high temperature.
When a high carbon dioxide concentration causes the oxyhaemoglobin curve to shift to the right meaning the affinity for oxygen decreases as the acidic carbon dioxide changes the shape of haemoglobin slightly. Heat also plays a role because it’s released during anaerobic respiration where there’d be a low partial pressure of oxygen and high partial pressure of carbon dioxide.
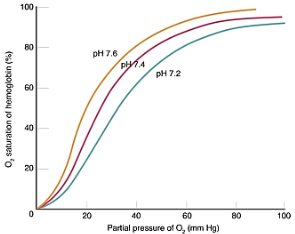
Describe what’s happening at lower and higher partial pressure in this oxygen dissociation curve showcasing the Bohr effect.
At low partial pressure of carbon dioxide in the alveoli, the curve shifts left, the affinity increases and so uploads more oxygen.
At high partial pressure of carbon dioxide at respiring tissues, the curve shifts right, the affinity decreases and so unloads more oxygen.
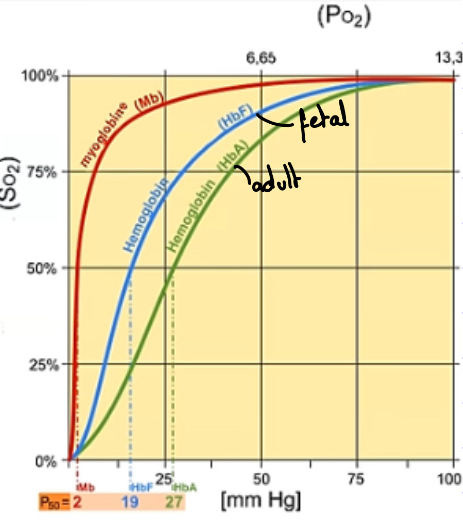
Describe what’s happening at lower and higher partial pressure in this oxygen dissociation curve showcasing fetal haemoglobin and why it’s an advantage.
Curve shifted to the left meaning a higher affinity because at the same partial pressure, haemoglobin is more saturated.
This is an advantage because it’s only source of oxygen is from the mother’s haemoglobin so it must have higher affinity for oxygen.
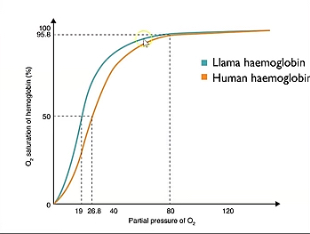
Describe what’s happening at lower and higher partial pressure in this oxygen dissociation curve showcasing llama haemoglobin and why it’s an advantage.
Curve shifted left meaning higher affinity.
This is an advantage because they’re found in higher altitudes where there’s lower partial pressure of oxygen so they must have higher affinity for oxygen.
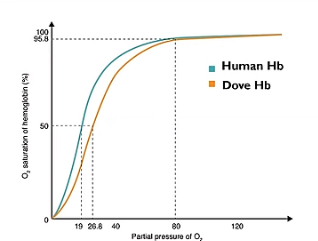
Describe what’s happening at lower and higher partial pressure in this oxygen dissociation curve showcasing dove haemoglobin and why it’s an advantage.
Curve shifted right so haemoglobin has a decreased affinity for oxygen meaning it’s more likely to be u loafing oxygen at the same partial pressure.
This is an advantage because doves have a very high metabolism so need a much higher supply of oxygen to continue respiration.
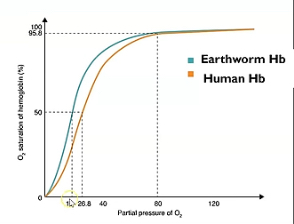
Describe what’s happening at lower and higher partial pressure in this oxygen dissociation curve showcasing earthworm haemoglobin and why it’s an advantage.
Curve shifted left meaning higher affinity for oxygen.
This is an advantage because they live underground where there’s a lower partial pressure of oxygen and therefore they require a higher affinity for oxygen.