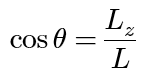Atomic Physics 1
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
shell
the collection of states with the same principal quantum number n (same energy)
subshell
the collection of states for which both the principal quantum number and azimuthal quantum number are the same
ionization energy
the energy required to remove the most loosely-bound electron from a neutral atom
electron affinity
the amount of energy released when an electron attaches to a neutral atom or molecule
atomic absorption/emission
an atom can transition from one energy state to another by emitting or absorbing light
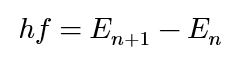
electron orbital angular momentum
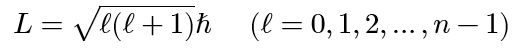
Planck’s constant(s)
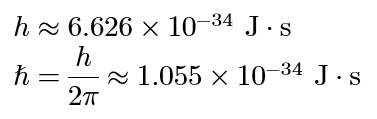
electron orbital angular momentum (z-component)
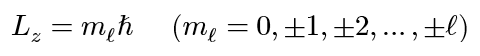
Bohr magneton
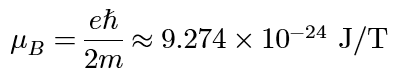
orbital magnetic moment of electron
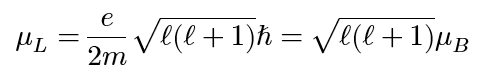
orbital magnetic moment of electron (z-component)
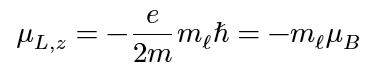
spin angular momentum of an electron
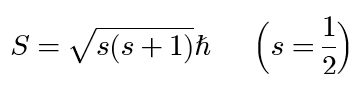
spin angular momentum of electron (z-component)
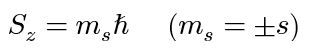
electron spin dipole moment
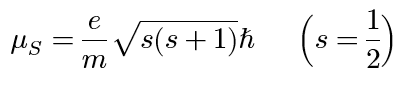
electron spin dipole moment (z-component)
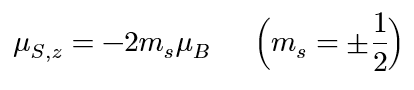
semi-classical angle