Electrochemistry
1/40
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Electrolyte
Those substances which dissociate into ions in their aqueous solution on passing electricity are known as electrolytes
Eg: NaCl, KCl
Strong Electrolyte
which dissociate completely into ions
e.g. HCl, HNO₃, H₂SO₄, etc.
Weak Electrolyte
which dissociate partially into ions
e.g. NH₄OH, Ca(OH)₂, CH₃COOH, etc.
Cell
It is a device used to convert one form of energy into another form of energy
Electrochemical Cell
It is an apparatus that changes chemical energy into electrical energy
Anode is -ve
Cathode is +ve
Spontaneous redox reaction occurs
Does not require an external voltage source
Made up of two electrolytes
A salt bridge is required to connect to half cells
Electricity is produced
WHen it works, mass of anode reduces and mass of cathode increases
Remember as LOAN (Left-side Oxidation Anode Negative)
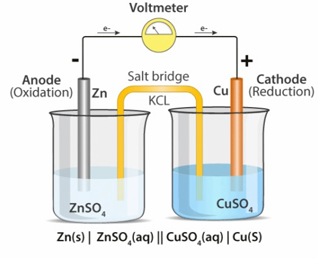
Salt Bridge
Salt bridge is an inverted U-shaped tube which internally connects the two half cells of an electrochemical cell
It contains a strong electrolyte dissolved in glycerol or gelatin
Significance:
Maintains the electrical neutrality of an electrochemical cell
It allows exchange of ions between two half cells and avoids mixing up of electrolye
It internally completes the electrical circuit
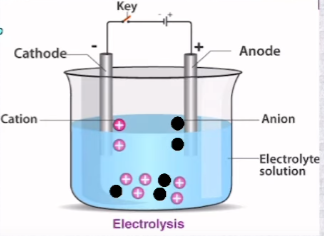
Electrolytic Cell
It is an apparatus that changes electrical energy into chemical energy
Anode is +ve
Cathode is -ve
Non-Spontaneous electro decomposition occurs
Requires an external voltage source
Made up of only one electrolyte
A salt bridge is not required
Electricity is consumed
Remember as PANIC (Positive Anode Negative In Cathode)
Electrode Potential (E)
The potential difference developed between the electrode and the electrolyte
Standard Electrode Potential (Eₒ)
The electrode potential of an element measured at 298K, 1 bar when the electrode is dipped in a unit molar electrolyte
Cell Potential (E(cell))
The potential difference between the two electrodes
It is called the cell electromotive force (emf) of the cell when no current is drawn through the cell
E(cell) = E(right)-E(left) = E(cathode) - E(anode)
If Eₑₓₜ > E°(cell) is supplied, the electrochemical cell becomes an electrolytic cell
If E°(cell) < 0, the cell is an electrolytic cell
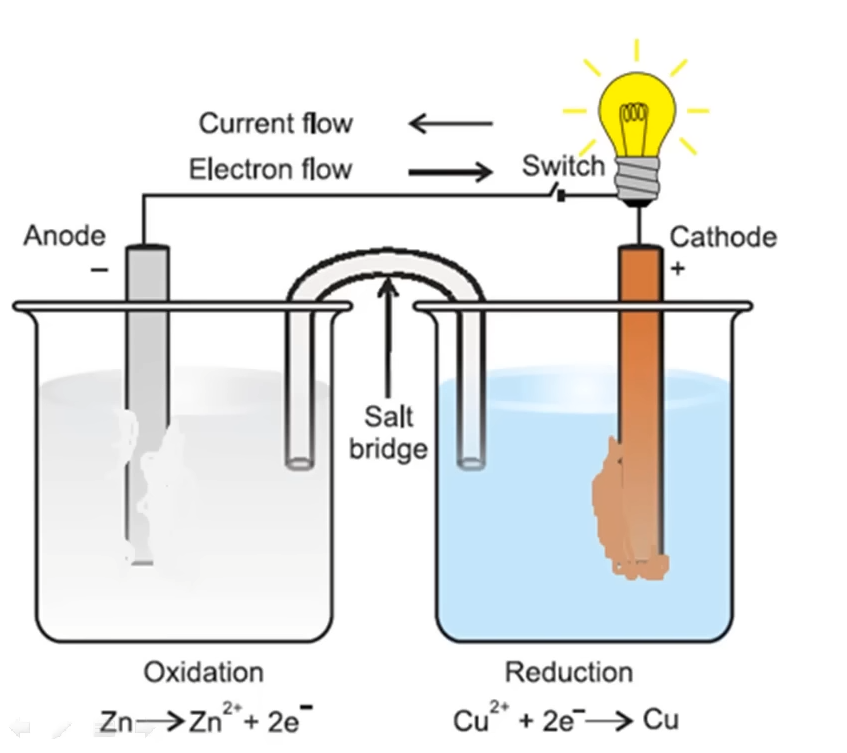
Galvanic Cell Representation
Cell Representation
Zn(s) / Zn²⁺(aq) // Cu²⁺(aq) / Cu(s)
Cell Reaction:
Zn + Cu²⁺ → Zn²⁺ + Cu
Half-cell Reactions:
Cathode (reduction): Zn → Zn²⁺ + 2e⁻
Anode (oxidation): Cu²⁺ + 2e → Cu⁻
Cell Potential:
E(cell) = E(Cu²⁺/Cu) - E(Zn/Zn²⁺)
Standard Hydrogen Electrode (SHE)
The standard hydrogen electrode consists of a platinum electrode coated with platinum black
The electrode is dipped in an acidic solution and pure hydrogen gas is bubbled through it
The concentration of hydrogen ion solution is one molar
Used to measure the electrode potential of a half-cell
If the standard electrode potential of an electrode is greater than zero then its reduced form is more stable compared to hydrogen gas.
Similarly, if the standard electrode potential is negative then hydrogen gas is more stable than the reduced form of the species.
The smaller the E° value, the greater the reducing power, easier to lose e⁻
The greater the E° value, the greater the oxidising power, and easier to gain e⁻
Nernst Equation
Mathematical relation between electrode potential and concentration of electrolyte
The Nernst equation for a single electrode reduction potential for a reduction reaction
Mn+ + ne– → nM
Ered = E(Mn+/ M) = E°(Mn+/M) – (0.0591/n) log(1/[Mn+])
For a cell reaction,
E(cell) = E°(cell) - (0.0591/n) log([Products]/[Reactants])
E(cell) = E°(cell) - (0.0591/n) log([O]/[R])
E(cell) = E°(cell) - (0.0591/n) logK𝒸
Work Done by Cell
∆G = -nFE(cell)
∆G° = -nFE°(cell)
∆G => Gibb’s free energy (amount of useful work done)
F => Faraday constant (charge on 1 mol of e⁻) = 96500Cmol⁻¹
For a feasible working cell:
∆G = -ve
E(cell) = +ve
Nernst Equation based on Gibb’s Free Energy
∆G° = -2.303RTlogK𝒸
∆G° = -nFE°(cell)
Metallic Conductors
The flow of electricity is due to the flow of electrons.
Also known as electronic conductors.
The flow of electricity takes place without the decomposition of a substance.
The electrical conduction decreases with temperature increase because kernels start vibrating, which hinders the flow of electrons.
Low as well as high voltage of current can pass through metallic conductors.
Certain materials called superconductors by definition have zero resistivity or infinite conductivity.
Earlier, only metals and their alloys at very low temperatures (0 to 15 K) were known to behave as superconductors, but nowadays several ceramic materials and mixed oxides are also known to show superconductivity at temperatures as high as 150 K
e.g. Fe, Al, Ag etc.
Electrolytic Conductors
The flow of electricity is due to the flow of ions.
Also known as ionic conductors.
The conductance of electricity by ions present in the solutions is called electrolytic or ionic conductance
The flow of electricity takes place by the decomposition of a substance.
The electrical conduction increases with the increase in temperature because the increase of temperature increases ion dissociation or decreases interionic attraction.
Only low voltage current can pass through electrolytic conductors.
e.g. NaCl, NaOH etc.
Factors Affecting Ionic Conductance
The nature of the electrolyte added
The size of the ions produced and their solvation
The nature of the solvent and its viscosity
Concentration of the electrolyte
Temperature (it increases with the increase of temperature)
Note: Ionic conductance increases with increase in temperature whereas electronic conductance decreases with increase in temperature
Resistance of Electrolytic Solutions (R)
R = ρ l/A
R = ρG*
G* (cell constant) = l/A
Conductance of Electrolytic Solutions (G) and Conductivity (K)
The reciprocal of resistivity is known as specific conductance or simply conductivity.
G = 1/R
K = 1/ρ
G = K A/l
G = K/G*
Molar Conductivity
Λₘ = K*1000/c Scm²mol⁻¹
Limiting Molar Conductivity
Molar conductivity at infinite dilution or maximum molar conductivity when concentration tends to be 0
Variation of Conductivity and Molar Conductivity with Concentration
Conductivity decreases with a decrease in concentration for both weak and strong electrolytes
Molar conductivity increases with a decrease in concentration
Λₘ = KV
As volume increases, Λₘ also increases
For strong electrolytes, Λₘ increases slowly with dilution and can be represented by the equation:
Λₘ = Λ°ₘ - Ac¹/²
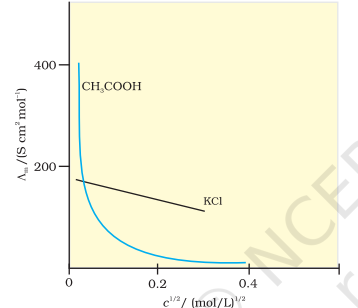
Constant A
The value of the constant ‘A’ for a given solvent and temperature depends on the type of electrolyte i.e., the charges on the cation and anion produced on the dissociation of the electrolyte in the solution
Thus, NaCl, CaCl₂ , MgSO₄ are known as 1-1, 2-1 and 2-2 electrolytes respectively. All electrolytes of a particular type have the same value for ‘A’.
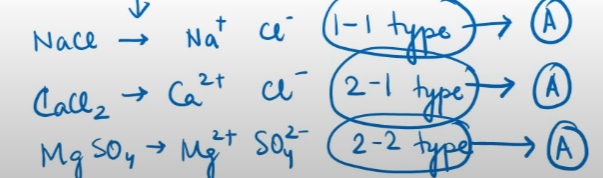
Kohlrausch Law of Independent Migration of Ions
(For Strong Electrolytes)
The law states that limiting the molar conductivity of an electrolyte can be expressed as the sum of the limiting molar conductivity of the individual ions in the electrolyte Λ°ₘ(CA) = λ°ₘ(Cⁿ⁺) + λ°ₘ(Aⁿ⁻)
Λ°ₘ(XaYᵦ) = a λ°ₘ(Xᵇ⁺) + bλ°ₘ(Xᵃ⁻)
Λ°ₘ(CA)= Λ°ₘ(CY) + Λ°ₘ(XA) - Λ°ₘ(XY)
Applications of Kohlrausch Law
To find Λ°ₘ of weak electrolyte
To find degree of dissociation of weak electrolyte
α = Λₘ/Λ°ₘ
To find dissociation constant
K𝒸 = α²c/1-α
Electrolytic Cell
It is an apparatus that changes electrical energy into chemical energy
Anode is +ve
Cathode is -ve
Non-Spontaneous electro decomposition occurs
Requires an external voltage source
Made up of only one electrolyte
A salt bridge is not required
Electricity is consumed
Remember as PANIC (Positive Anode Negative In Cathode)
Copper Purification
One of the simplest electrolytic cells consists of two copper strips dipping in an aqueous solution of copper sulphate.
If a DC voltage is applied to the two electrodes, then Cu²⁺ ions discharge at the cathode (negatively charged) and the following reaction takes place:
Cu²⁺(aq) + 2e⁻ → Cu (s)
Copper metal is deposited on the cathode. At the anode, copper is converted into Cu²⁺ ions by the reaction:
Cu(s) → Cu²⁺(s) + 2e⁻
Thus copper is dissolved (oxidised) at the anode and deposited (reduced) at the cathode.
This is the basis for an industrial process in which impure copper is converted into copper of high purity.
The impure copper is made of an anode that dissolves on passing current and pure copper is deposited at the cathode.
Faraday’s First Law
(a) A current of 1.50 A was passed through an electrolytic cell containing AgNO₃ solution with inert electrodes. The weight of Ag deposited was 1.50 g. How long did the current flow?
(b) Write the reactions taking place at the anode and cathode in the above cell.
(c) Give the reactions taking place at the two electrodes if they are made up of Ag.
The amount of substance liberated at an electrode of an electrolytic cell is directly proportional to the quantity of electric charge passed through the electrolyte
w ∝ Q
w ∝ It
w = ZIT
w = MᵣIt/nF
Z (electrochemical equivalent) = E/F = Mᵣ/nF
w=> mass of copper deposited
A: https://www.toppr.com/ask/question/a-a-current-of-150a-was-passed-through-an-electrolytic-cell-containing-agno/
Faraday’s Second Law
When the same amount of current is passed through two different electrolytes, the mass deposited at the respective electrodes is directly proportional to the equivalent weight of the substance
w ∝ E
E => equivalent weight (Atomic Mass of Metal ÷ Number of electrons required to reduce the cation)
w₁/w₂ = E₁/E₂
w₁/w₂ = (M₁/n₁) x (n₂/M₂)
Product of Electrolysis
1) Write the reaction occurring at the anode and cathode and the products of electrolysis of aq. NaCl.
2) Assertion (A) : Electrolysis of aqueous solution of NaCl gives chlorine gas at anode instead of oxygen gas.
Reason (R) : Formation of oxygen gas at anode requires overpotential. (answer is a)
see cw

Battery
One or more cells connected in series
A galvanic cell where the chemical energy of the redox reaction is converted to electrical energy
Primary Batteries
Those batteries which cannot be rechargeable are known as primary batteries.
Eg: Dry cell, Mercury cell
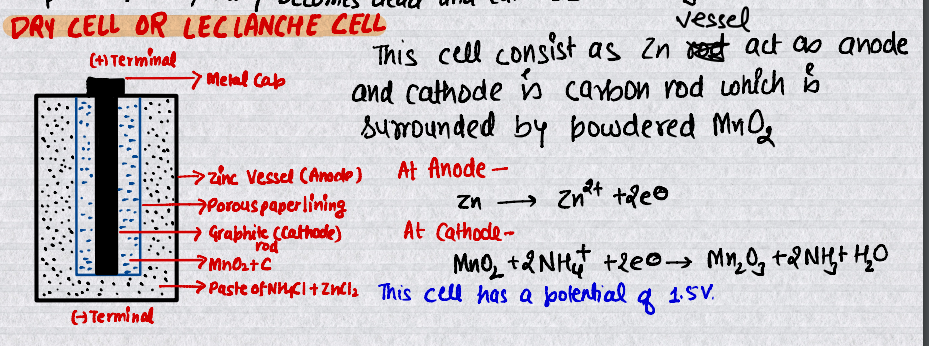
Dry Cell (Leclanche Cell)
The most familiar example of this primary battery is the dry cell (Leclanche cell after its discoverer) commonly used in our transistors and clocks.
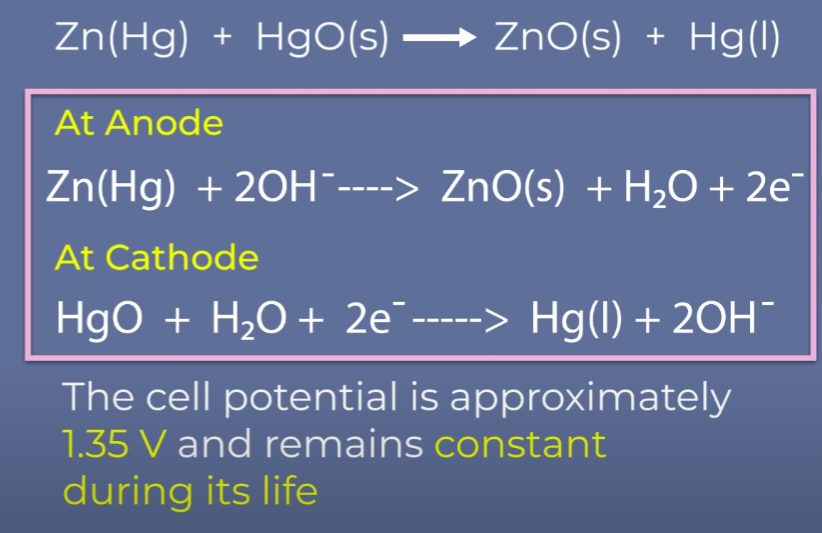
Mercury Cell
Why does the cell potential of mercury cell remain constant throughout its life?
Mercury cells are suitable for low-current devices like hearing aids, and watches.
A: Because no ions are involved in the cell reaction.
Secondary Batteries
A secondary cell after use can be recharged by passing a current through it in the opposite direction so that it can be used again
Eg: Lead Storage Battery
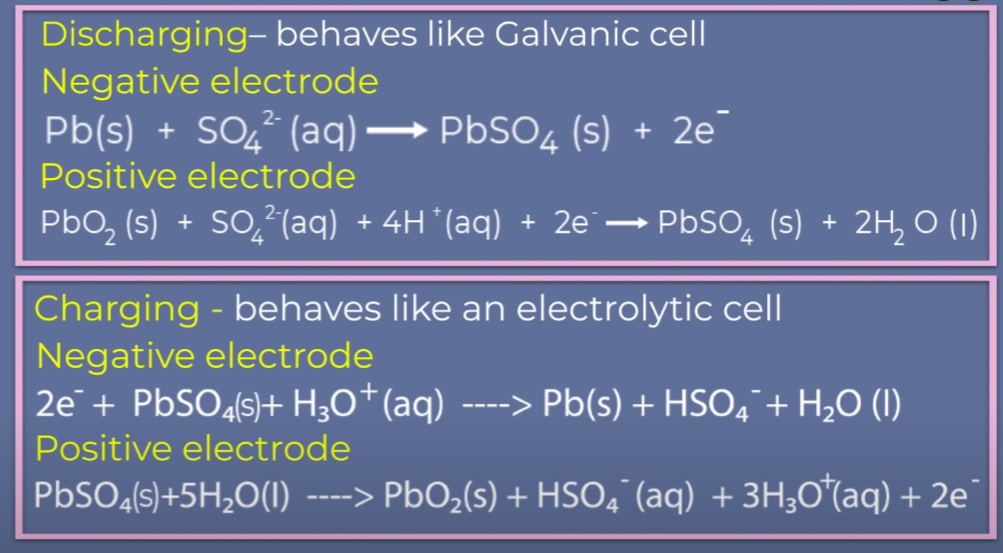
Lead Storage Battery
The most important secondary cell is the lead storage battery commonly used in automobiles and invertors. It consists of a lead anode and a grid of lead packed with lead dioxide (PbO2 ) as cathode. A 38% solution of sulphuric acid is used as an electrolyte.
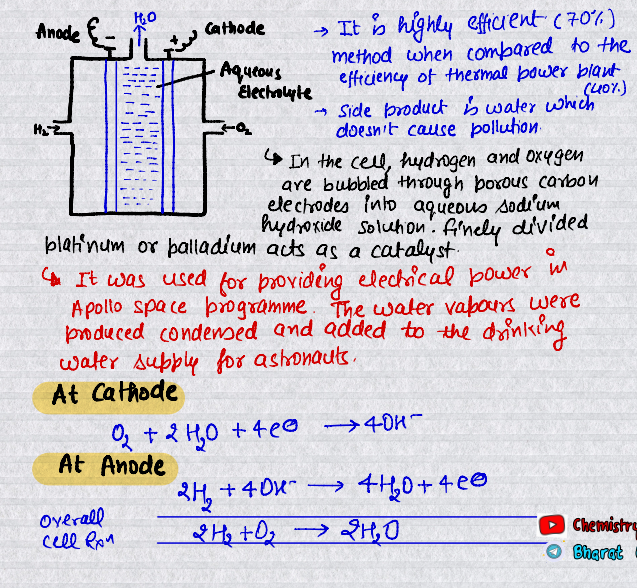
Fuel Cells
Galvanic cells that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into electrical energy are called fuel cells.
It is pollution-free and highly efficient
Corrosion
Corrosion slowly coats the surfaces of metallic objects with oxides or other salts of the metal.
Eg: rusting of iron, tarnishing of silver, development of green coating on copper and bronze
Rusting of Iron
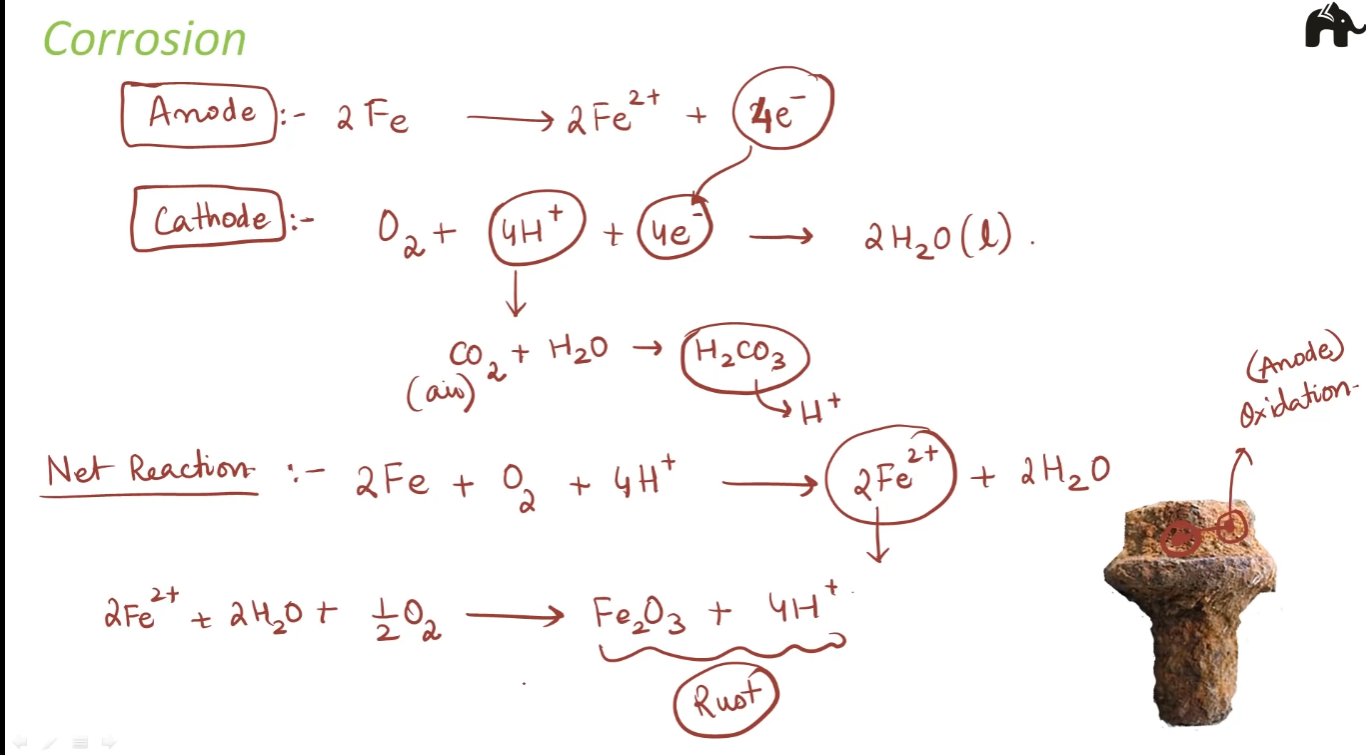
Prevention of Corrosion
Prevent corrosion by blocking contact between the metal surface and the atmosphere by covering the metal surface with paint or chemicals (e.g., bisphenol).
Use a protective layer of inert or reactive metals (e.g., Sn, Zn) to safeguard the object.
Use a sacrificial electrode (e.g., Mg, Zn) that corrodes to protect the main object.