exam 1
1/57
Earn XP
Description and Tags
learning objectives (unit 1 - lsn 1-5)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
58 Terms
describe the contributions of Antoni van Leeuwenhoek to the field of microbiology
originally a textile merchant - used lenses to inspect fabric quality
created simple microscopes (did NOT create the first microscope)
KNOWN FOR: discovering bacteria (tiny animals - animalcules) and documenting them
will be later called microorganisms
list and describe six groups of microorganisms and differentiate prokaryotic from
eukaryotic microorganisms
prokaryotes (no defined nucleus, usually smaller than eukaryotes): bacteria and archaea
eukaryotes (defined nucleus, usually larger than prokaryotes): fungi. protozoa, algae, and helminths
bacteria
unicellular and lack nuclei
much smaller than eukaryotic cells
found anywhere moisture exists
reproduces asexually
peptidoglycan in cell walls
some lack a cell well altogether
archaea
unicellular and lack nuclei
much smaller than eukaryotic cells
found anywhere moisture exists
reproduces asexually
cell walls are made from different polymers
lives in extreme environments (hot springs)
fungi
eukaryotic (has membrane bound organelles)
heterotrophic: obtains food from other organisms
cell walls composed of chitin
molds
multicellular
grows as long filaments
reproduce by sexual and asexual spores
yeasts
unicellular
reproduce asexually by budding
some produce sexual spores
protozoa
single-celled eukaryotes
lack a cell wall
animal-like nutrients needs and structure
live freely in water or inside animal hosts
pseudopods: false feet, flowing extensions of cytoplasm (crawling movements)
cilia: many short, hair-like structures for swimming
flagella: few long, whip-like tails for propulsion
algae
vary widely in size, shape, pigmentation, and structure
unicellular or multicellular
photosynthetic (uses sunlight for food)
simple reproductive structures
classification: based on pigmentation and cell wall composition
helminths
compare and contrast the investigations of Redi, Needham, and Spallanzani
concerning spontaneous generation (life arising from nonliving matter)
Redi’s experiment
designed a direct test of spontaneous generation
meat kept isolated from flies never developed maggots
meat exposed to flies became infested with maggots
maggots arose from fly eggs, not the meat itself
challenges Aristotle’s theory and case the first serious doubt on spontaneous generation
Needham’s experiment
tested spontaneous generation
boiled beef gravy and plant infusions briefly (did not sterilize)
sealed flasks with corks after boiling (cork is permeable to air)
microbes still appeared in sealed flasks
life must arise from nonliving matter-possibly via a “life force”
results seemed to support Aristotle’s view - that microbes could arise from nonliving matter
Spallanzani’s experiment
challenged Needham’s conclusions
boiled broth for one hour (sterilized)
sealed flasks by melting the necks or using wax (not permeable to air)
no growth unless flasks were exposed to air
strong evidence against spontaneous generation
showed that air exposure, not “life force” was necessary for microbial growth
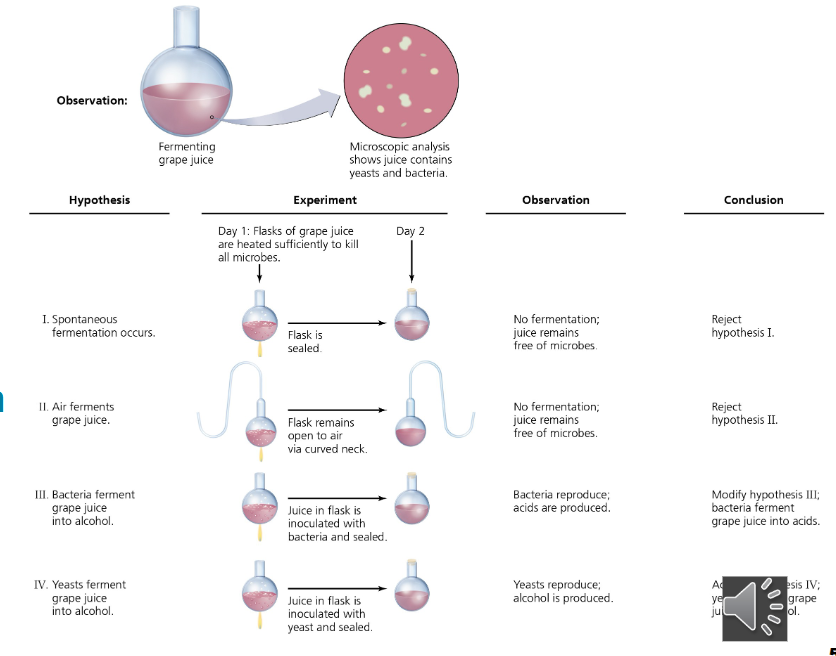
describe the contributions of Louis Pasteur to the field of microbiology relative to
spontaneous generation
used swan-shaped necks to trap airborne particles
flasks left upright showed no microbial growth (outside air could not reach the flask)
when tilted, dust from the neck entered the broth, cloudy with microbes in 1 day
microbes came from the environment, not spontaneous generation
Louis Pasteur to the field of microbiology relative to fermentation
spoiled wine threatened the livelihoods of many grape growers, wine produces funded research to promote alcohol production and production and prevent spoilage during fermentation, cause of fermentation reactions become linked to the broader debate over spontaneous generation
demonstrated that heating liquids could kill most microbes without ruining flavor
developed the process of pasteurization
founded the filed of industrial microbiology - intentional use of microbes to manufacture products
Eduard Buchner’s biochemistry
scientists still debated whether cells themselves were required for fermentation
his experiment showed fermentation could occur without living cells
demonstrated that enzymes (cell-free extracts) drive chemical reactions
proved biological reactions can occur outside cells
marked the beginning of modern biochemistry
Louis Pasteur to the field of microbiology relative to germ theory of disease
people believed that bad air (miasma) caused disease
from Hippocrates
claimed disease arose from “noxious air”
Pasteur proposed the germ theory of disease
Robert Koch provided experimental proof using isolated microbes
showed that specific microorganisms cause specific diseases
pathogens
list and describe the steps of the scientific method
observation: notice something unusual
question: ask a specific question based on the observation
hypothesis: propose a testable explanation
experiment: test the hypothesis through controlled experiments
distinguish between hypothesis and theory
hypothesis: a proposed explanation for a phenomenon, formulated as a testable statement that can be supported or refuted through experimentation and observation
theory: a well-substantiated explanation of some aspect of the natural world that is supported by a body of evidence and has withstood multiple tests through scientific methods
Describe Robert Koch’s contributions to the field of microbiology
developed simple staining techniques
created the first photomicrograph of bacteria
took the first photograph of bacteria in diseased tissue
devised methods for estimating bacterial numbers in solutions
introduced steam sterilization of growth media
popularized the Petri dish (invented by Julius Petri)
refined lab transfer techniques for culturing bacteria
recognized bacteria as distinct species
identify each of the four steps that compose “Koch’s postulates”
scientists lacked proof that microbes were responsible for specific diseases
studied causative agents of disease (etiology - study of the cause of disease)
demonstrated that a bacterium causes anthrax
used microbial colonies to like individual microbes to particular diseases
suspected agent must be found in every case of disease and absent from healthy hosts
agent must be isolated and grown outside the hose (pure culture)
when the agent is introduced into a healthy, susceptible host, the host must get the disease
same agent must be re-isolated from the newly diseased host
formed the basis for linking specific microbes to specific diseases
apply basic chemistry concepts to the study of microbiology: atomic structure, isotopes, and electron configurations
atomic structure
atomic number = number of protons (change this number = change the element)
atomic mass = protons + neutron (atomic mass units - amu), electrons have no mass
isotopes = atoms with the same number of proton but different numbers of neutrons
electron configurations
complex 3D shapes that describe where electrons most likely to be found
orbitals represent spatial probability zones, not fixed paths
valence = the ability of an atom to bond with others - lose, gain or share

apply basic chemistry concepts to the study of microbiology: molecules vs. compounds
molecule = two or more atoms held together by chemical bonds
compound = a molecule made of different elements
apply basic chemistry concepts to the study of microbiology: covalent bonding vs. ionic bonding
covalent bond = sharing a pair of electrons between atoms
ionic bond = formed when electrons are transferred (difference in electronegativity >, or equal to 2.0)
apply basic chemistry concepts to the study of microbiology: nonpolar vs. polar covalent bonds
nonpolar covalent bonds = electrons are shared equally, resulting in no charge (difference in electronegativity 0.0 - 0.4)
polar covalent bonds = electrons are shared unequally, pulled more toward one atom (difference in electronegativity 0.5 - 1.9)
apply basic chemistry concepts to the study of microbiology: hydrogen bonds
hydrogen bonds = electrostatic attractions between a partially positive hydrogen and a partially or fully negative atom (often O or N)
weaker than covalent bonds - important in biological structures
stabilize 3D structures of large molecules
allow DNA strands to separate and rejoin during replication/transcription
give an example of a synthesis reaction, decomposition reaction, and exchange reaction
synthesis = a chemical process where two or more simple substances chemically bond to form a single, more complex product
decomposition = a chemical change where one complex compound breaks down into two or more simpler substances
exchange = a chemical reaction in which both synthesis and decomposition occur, chemical bones are both formed and broken, and chemical energy is absorbed, stored, and released
describe the properties of water: cohesion, adhesion, solvent, high specific heat capacity
cohesion = water molecules stick to each other
caused by hydrogen bonding
responsible for surface tension (why droplets form and bugs can walk on water)
adhesion = water molecules stick to other surfaces, especially polar or charged ones
helps water cling to glass, plant tissue, and paper
drives capillary actions (ex: water climbing a plant stem)
high specific heat capacity
the amount of energy needed to convert 1 gram of liquid into gas
high heat of vaporization due to hydrogen bonding
helps regulate temperatures in ecosystems and our bodies
describe the properties of water: role of water in biochemical reactions
role of water in biochemical reactions
dissolve many substances, especially ions and polar molecules
water is polar, allowing it to surround and separate charged particles
solvent (water) = substance doing the dissolving
solute (ex: NaCl) = substance being dissolved
universal solvent makes it essential for transport, metabolism, and cellular function
solubility drives everything from blood chemistry to nutrient absorption
contrast acids, bases, and salts and explain the role of buffers relative to pH change
acids: releases H+ (protons) into solution)
more H+ → lower pH (acidic)
base: either binds to H+ or releases OH-
more OH- → higher pH (basic)
acids donate protons, bases accept them
pH is the power of hydrogen
buffers = resist sudden changes in pH by neutralizing small amounts of added acid or base
ex: bicarbonate buffer system keeps blood pH around 7.4
most biochemical reactions only work properly within a narrow pH range
metabolism depends on maintaining a stable acid-base balance
different animals have different pH tolerances (ex: 6.5-8.5)
list the four classes of biological macromolecules and understand their synthesis through dehydration synthesis reactions and breakdown through hydrolysis reactions
carbohydrates - quick energy and structural support
proteins - catalysts, cell structure, and signaling molecules
lipids - energy storage, membranes, and hormones
nucleic acids - genetic blueprints
all macromolecules are organic - they contain carbon
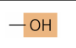
functional group - hydroxyl
—OH
polar, forms H-bonds
alcohols, sugars
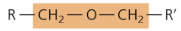
functional group - ether
—O—
links sugar units
disaccharides

functional group - ketone
C=O (internal)
reactive carbonyl
sugars

functional group - aldehyde
—CHO (terminal)
reactive carbonyl
sugars
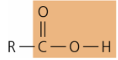
functional group - carboxyl
—COOH
acidic, donates H+
amino acids, fatty acids

functional group - amino
—NH2
basic, accepts H+
amino acids
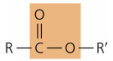
functional group - ester
—COOR
hydrophobic linkage
fats, waxes
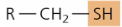
functional group - sulfhydryl
—SH
forms disulfide bridges
protein folding
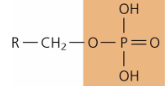
functional group - phosphate
—PO4
high energy, negative charge
ATP, nucleotides, phospholipids
functional group - methyl
—CH3
hydrophobic, nonpolar
lipids, gene regulation
discuss the roles of carbohydrates in living systems
functions:
ready energy (glucose, sucrose)
long-term energy storage (starch and glycogen)
form the backbone of nucleic acids (ribose and deoxyribose)
can be converted to amino acids
structural components (cellulose in plants and peptidoglycan in bacteria)
involved in cell recognition and signaling (glycoproteins/glycolipids)
recognize the basic structure of carbohydrates
monosaccharides - single sugar units (glucose, fructose)
disaccharides - two sugars bonded together (sucrose, lactose)
polysaccharides - long chains of sugars (starch, cellulose, glycogen)
glycosidic bond/linkage - bonds linking sugar
recognize the basic structure of carbohydrates (cont. OH configuration)
a-glucose - found in starch (digestible by humans)
OH on 1st carbon is below the plate
b-glucose - found in cellulose (not digestible by humans)
OH on 1st carbon is above the plate
N-acetylglucosamine
modified monosaccharide used in peptidoglycan (bacteria cell walls) and chitin (fungi, arthropods)
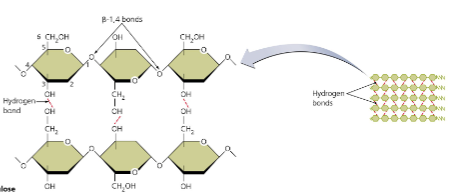
cellulose
straight chains, linked by β-1,4 bonds → structural support (plants, fiber)
not digestible by humans
branching increases energy accessibility
linear structures increase stability and
strength
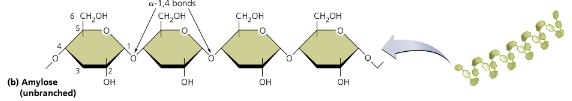
amylose (starch)
unbranched helical chains, linked by α-1,4 bonds → energy storage in plants
branching increases energy accessibility
linear structures increase stability and
strength
glycogen
highly branched chains, linked by α-1,4 and α-1,6 bonds → energy storage in animals
fast access to glucose
branching increases energy accessibility
linear structures increase stability and
strength
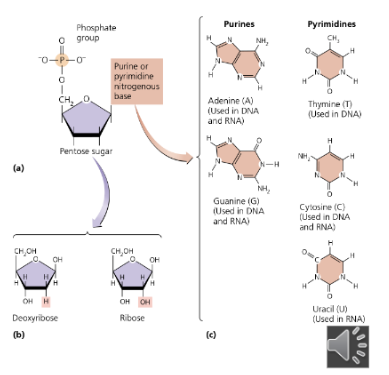
sketch and label the basic structure of a nucleotide - building blocks of nucleic acids
phosphate group
pentose sugar (ribose or deoxyribose)
nitrogenous base (A, T, G, C, or U)
nucleoside - sugar + base (no phosphate)
linked into a polymer by phosphodiester bonds
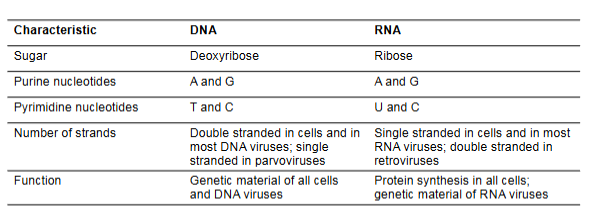
DNA vs RNA
DNA:
inheritance and genome stability
long-term genetic storage
deoxyribose sugar
contains thymine
antiparallel, complementary, 2 strands, stable (H on 2nd carbon)
RNA:
protein synthesis, regulation, catalysis
assists with gene expression, protein building, and sometimes acts as an enzyme
ribose sugar
contains uracil
more reactive (OH on 2nd carbon), one strand

nitrogenous base - pyrimidines
cytosine, thymine (DNA), uracil (RNA)
nitrogenous base - purines
adenine, guanine
ATP vs ADP vs AMP
contains:
ribose (sugar)
adenine (nitrogenous base)
triphosphate - 3 phosphate groups
diphosphate - 2 phosphate groups
monophosphate - 1 phosphate group
ATP
main short-term energy supply for cells
energy is release when phosphate bonds of ATP are broken
ATP supply is limited and must be replenished

sketch the basic structure of an amino acid
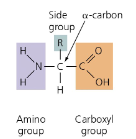
describe five general functions of proteins in living things
structure - keratin in skin and collagen in connective tissue
catalysis - enzymes accelerate chemical reactions
regulation - hormones and gene regulators (ex: insulin, repressors)
transport - channel and carrier proteins in cell membranes
defense and offense - antibodies, toxins, and complement proteins
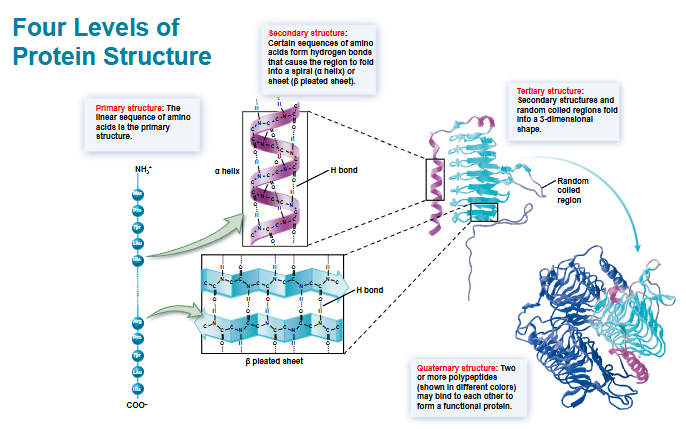
label the four levels of protein structure and elucidate five different factors that promote protein folding and stability
hydrogen bonds: Bonds form between atoms in the polypeptide backbone and between atoms in different side chains
ionic bonds: bones form between oppositely charged side chains
hydrophobic effect: nonpolar amino acids in the center of the protein avoid contact with water
van der Waals forces: attractive forces occur between atoms that are optimal distances apart
disulfide bridge: a covalent bond forms between 2 cysteine side chains
understand the structures and functions of lipids - triglyceride
linking three fatty acids to a glycerol backbone
long-term energy storage
saturated (no double bonds) vs. unsaturated (double bonds - 1 or more)
unsaturated bond - cis H is on same side - creating a kink
trans - H is on opposite sides - creating an unsaturated fat similar to saturated fats
understand the structures and functions of lipids - phospholipid
phosphate group + organic molecule
hydrophilic head, hydrophobic tail
kinks in unsaturated tails increase membrane fluidity
understand the structures and functions of lipids - waxes
1 long-chain fatty acid
linked to 1 long-chain alcohol
bonded via ester bond
lack a hydrophilic head, unlike phospholipid - totally water-insoluble
found in plants, insects, and animals
leaf cuticles to prevent water loss
glossy fruits
beeswax
waterproof barriers, flexible armor against desiccation and environmental damage
understand the structures and functions of lipids - steroids
cholesterol - nestle between phospholipids in membranes, helping maintain fluidity and stability
ridged ring structure interacts with fatty acid tails, reducing membrane permeability and preventing freezing or collapse
starting point for hormones - testosterone, estrogen, cortisol
are not chains, but rather rings = slip through membranes and bind to internal receptors, triggering targeted cellular responses
describe the four classes of biological macromolecules, understand their monomers, polymers, examples, and functions
REFER TO SLIDES