Fluid transport KNW 2
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
force types in fluid mech
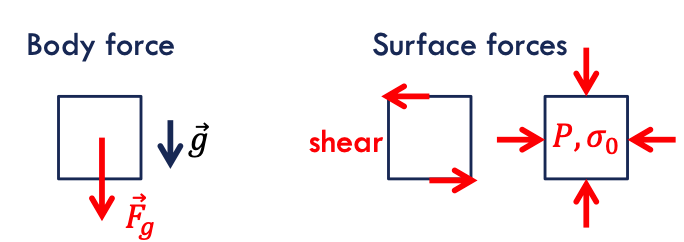
body forces → associated with the presence of external fields acting equally on all fluid elements
surface forces → acting on the surface of the control volume
reference systems
Lagrange → development of a fluid package of constant mass
Euler → mass flux through fixed volume
require local derivatives ads the property changes w/ time
navier stokes eq → consider the velocity field that changes as function of time and space
fluid types
inviscid = ideal fluids w/ no internal friction
viscous = fluids w/ friction (resistance to shear)
newtonian → µ = const
non-newtonian → shear rate dependant t viscosity
equations of motion
Euler equation: for ideal fluids considering gravity and normal stresses (pressure)
Navier-Stokes equation: considers in addition viscous forces for Newtonian fluids.
Stokes equation: derived from NS for stationary (steady state) flow.
hooks law
The force that is needed to extend or compress a spring is linear to the distance of elongation or compressions
stress types
static → amount of deformation
𝜎 = 𝑐 𝜀
𝜎 = 𝑁/𝑚2
𝜀 = 1
𝑐 = stiffness tensor
dynamic → rate of deformation
𝜏 = 𝜇 𝜀
𝜏 = 𝑁/𝑚2
𝜀 = 1/𝑠
viscous stress force types
Viscous forces with linear or volumetric dilation of fluid elements.
Apparent viscous forces associated with dissipation during 1. → related to bulk viscosity of a compressible fluid.
Surface forces associated with thermodynamic pressure
normal stress application cond
Static conditions → thermodynamic pressure → 𝜎𝑥 = 𝜎𝑦 = 𝜎𝑧
(arithmetic average)
Dynamic conditions → mechanical pressure → 𝝈𝒙 = 𝒑 + 𝝉𝒙𝒙
normal stress importance
they can occur under static conditions – isotropic (thermodynamic) pressure
They can occur under dynamic conditions:
In a deviatoric but purely non-deformable way (the principal shape is conserved) if the stresses are isotropic → bulk or volumetric viscosity
In a purely deformable way if the stresses are purely anisotropic → shear viscosity 𝜇.
heat capacity, C
the heat 𝑄 that is required to change the temperature by ∆𝑇
thermal diffusivity
measures the ability of a material to conduct thermal energy relative to its ability to store thermal energy
newtons 1st law of motion
an object either remains at rest or continues to move at a constant velocity 𝑢, unless acted upon by a force (constant momentum).
newtons 2nd law of motion
that rate of change of momentum of an object (body) is proportional to the net force applied to the object – the change is in direction of the applied force.
peclet number
dimensionless number describing the relative importance of advective to diffusive transport
Pe = ul/D
high Pe = advection dominated = fast flow
low Pe = diffusion dominated = slow flow
material balance
to ensure that we account for all mass within a system
accumulation = input + generation - output- consumption
input > output = accumulation
input < output = depletion
fick’s 1st law
the diffusive flux is proportional to the concentration gradient
J = -D ∂C/∂x
fick’s 2nd law
how concentration changes over time
∂C/∂t = D∇^2𝐶
advection
bulk transport due to fluid flow (how much of the bulk flow leaves/ enters the observed control volume
∂C/∂t + u ∇C = 0
diffusion
gradient driven spreading process
Why Dimensionless numbers?
Compare physical effects (e.g., advection vs. diffusion)
Generalize behavior across systems of different scales
Reduce the number of parameters in simulations and experiments
Damköhler number
describes the relative dominance of reaction vs advection (I) or diffusive (II)
Da I = Akl/v
Da II = Akl²/D
k… reaction rate const
D…diffusion coefficient
Da « 1 = transport dominates, reaction is slow
Da » 1 = reaction is fast relative to transport
energy balance equation
describes the conservation of energy within a system
∆E = (Qin - Qout) + (Win + Wout) + (Uin - Uout)