Chem 261 Vocabulary
1/76
Earn XP
Description and Tags
Copy from mc on quizlet
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
77 Terms
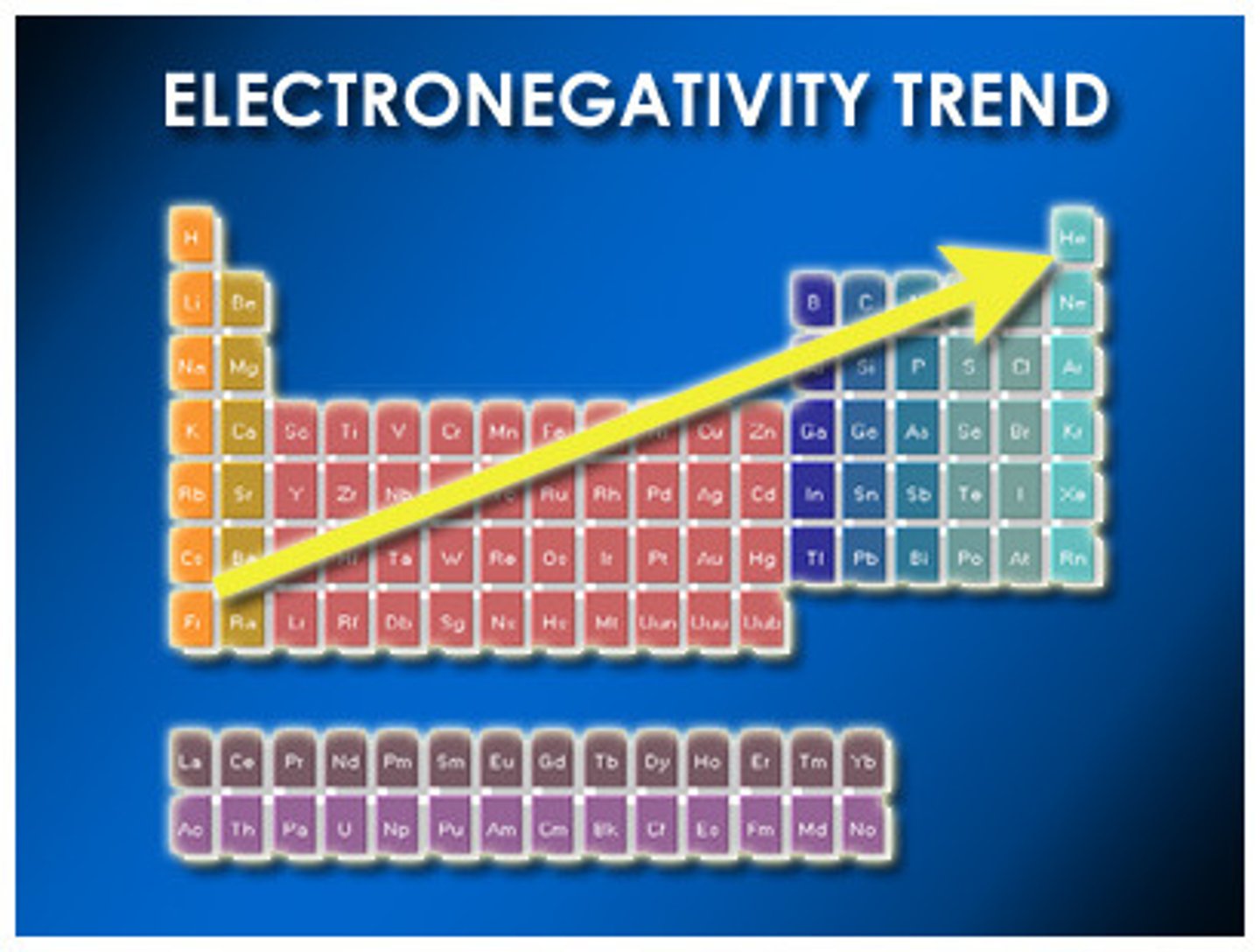
electronegativity
A measure of the ability of an atom in a chemical compound to attract electrons
bond lengths
the distance between two covalently bonded nuclei
bond angle
the angle formed between two covalent bonds
bond dissociation energy
the energy required for homolytic cleavage of a bond (covalent bonds)
heterolytic cleavage
cleavage of a bond to give a cation and an anion; one atom which is part of the covalent bond retains both electrons from the bond
dispersion forces
everything has these; most important in nonpolar covalent bonds, strength of dispersion forces is related to molecular weight and polarizability
condensed structural formula
structural molecular formula showing the general arrangement of atoms but without showing all the covalent bonds
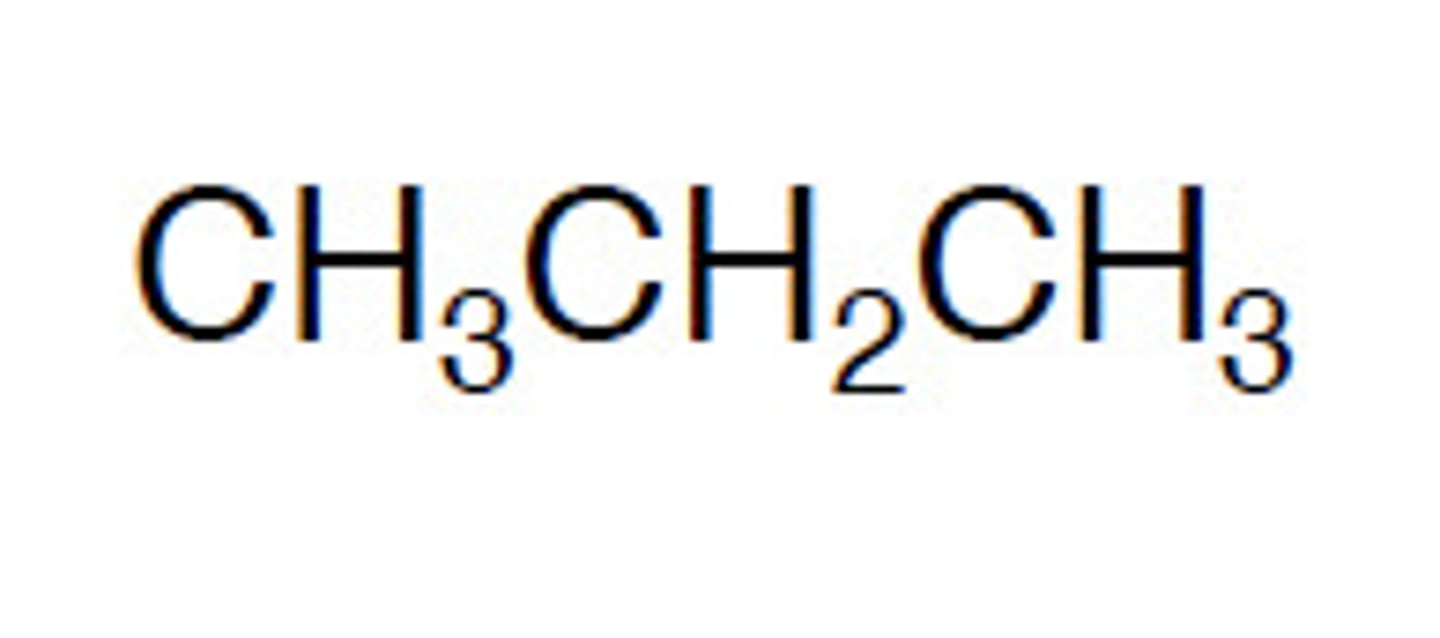
line-bond formula (aka skeletal structures)
a line is used to represent two electrons forming a bond (a shared pair)
condensed formula
bonds are always not shown and atoms of the same type bonded to another atom are grouped together
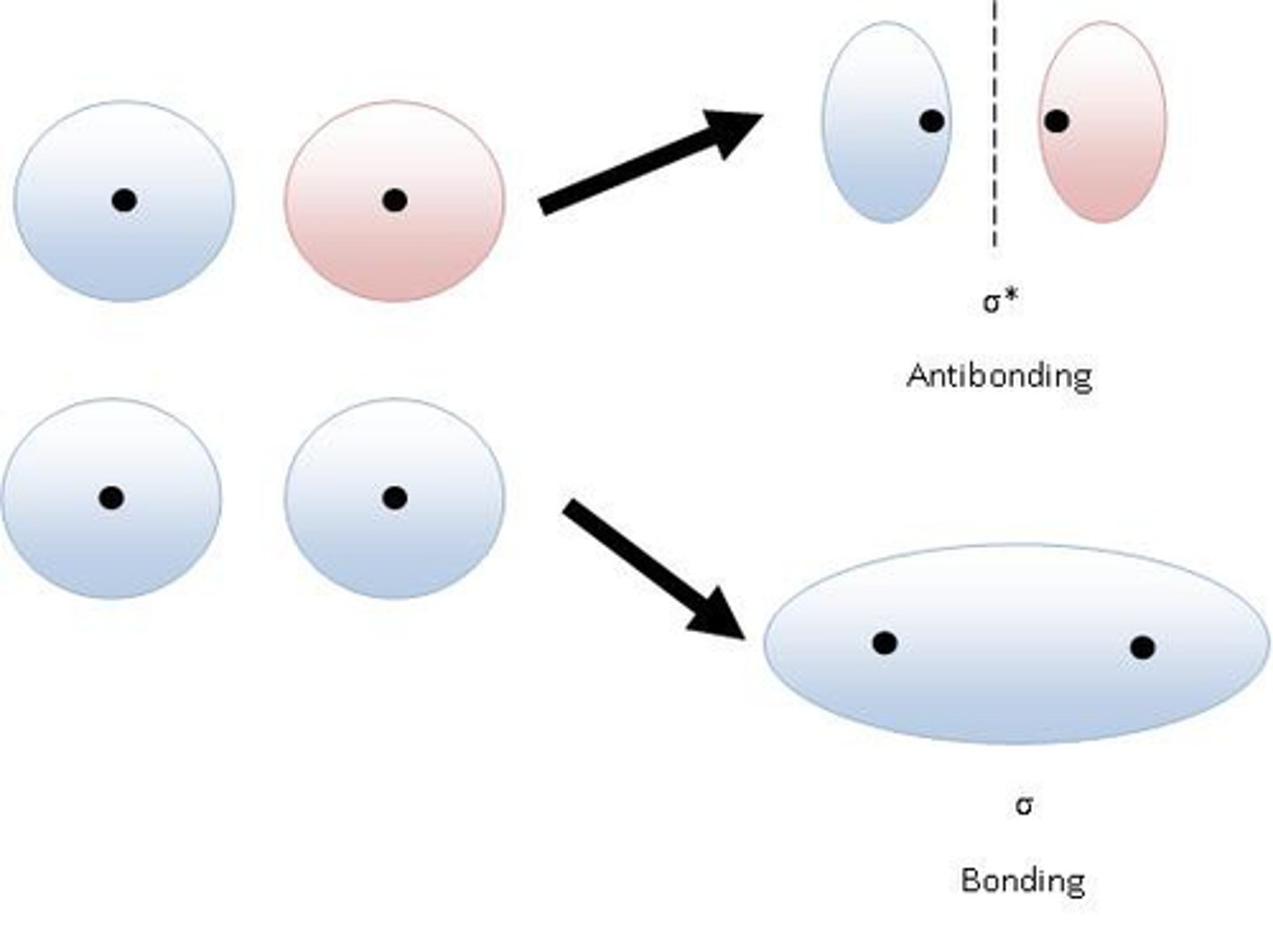
bond
in-phase waves; constructive interference (think "combine")
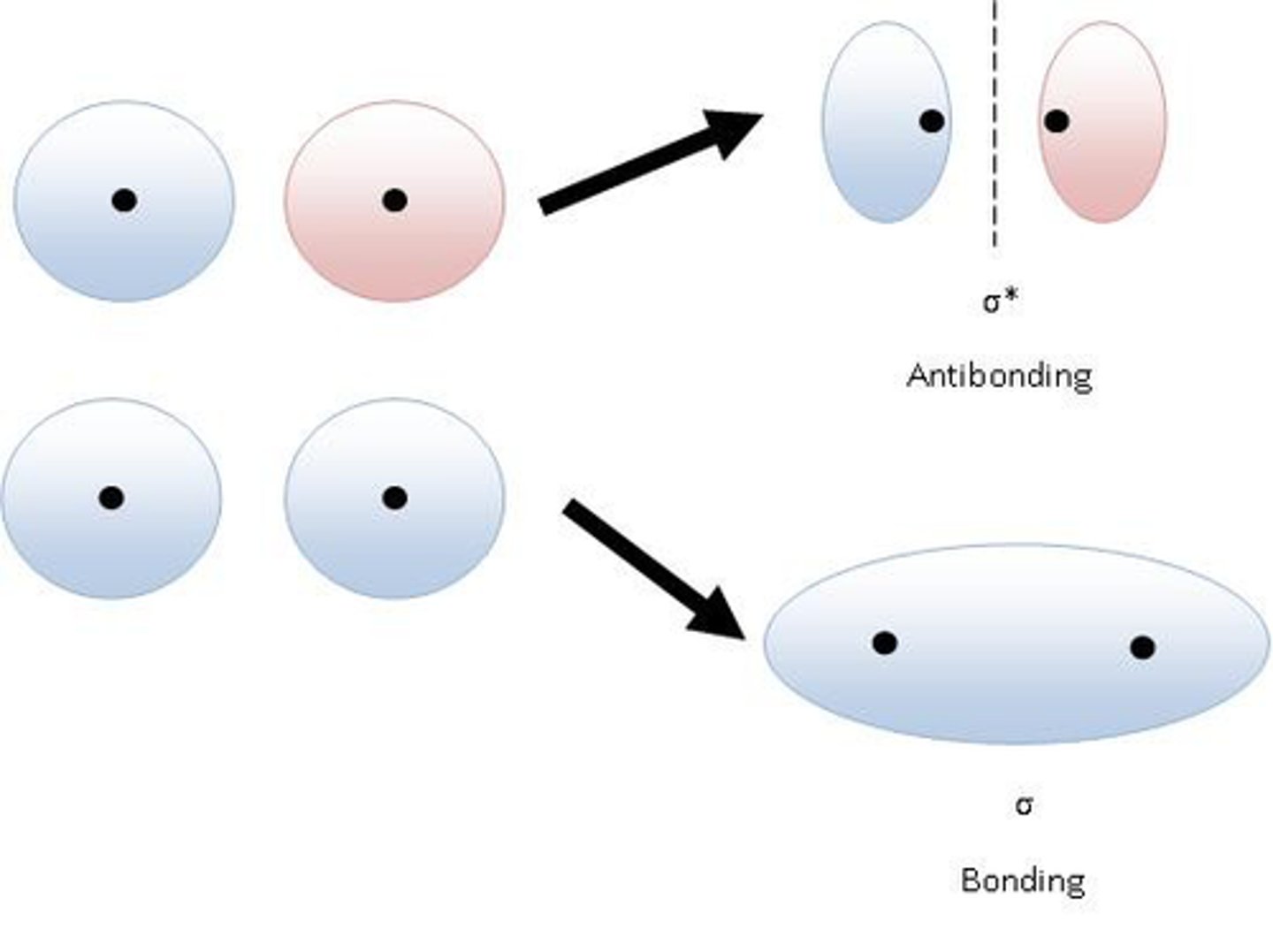
antibonding
out-of-phase waves; destructive interference (think "cancel")
node
point at which the amplitude is zero; shaded space within an orbital; no electron density here
antibonding orbital (𝜎*)
node between nuclei, zero electron density
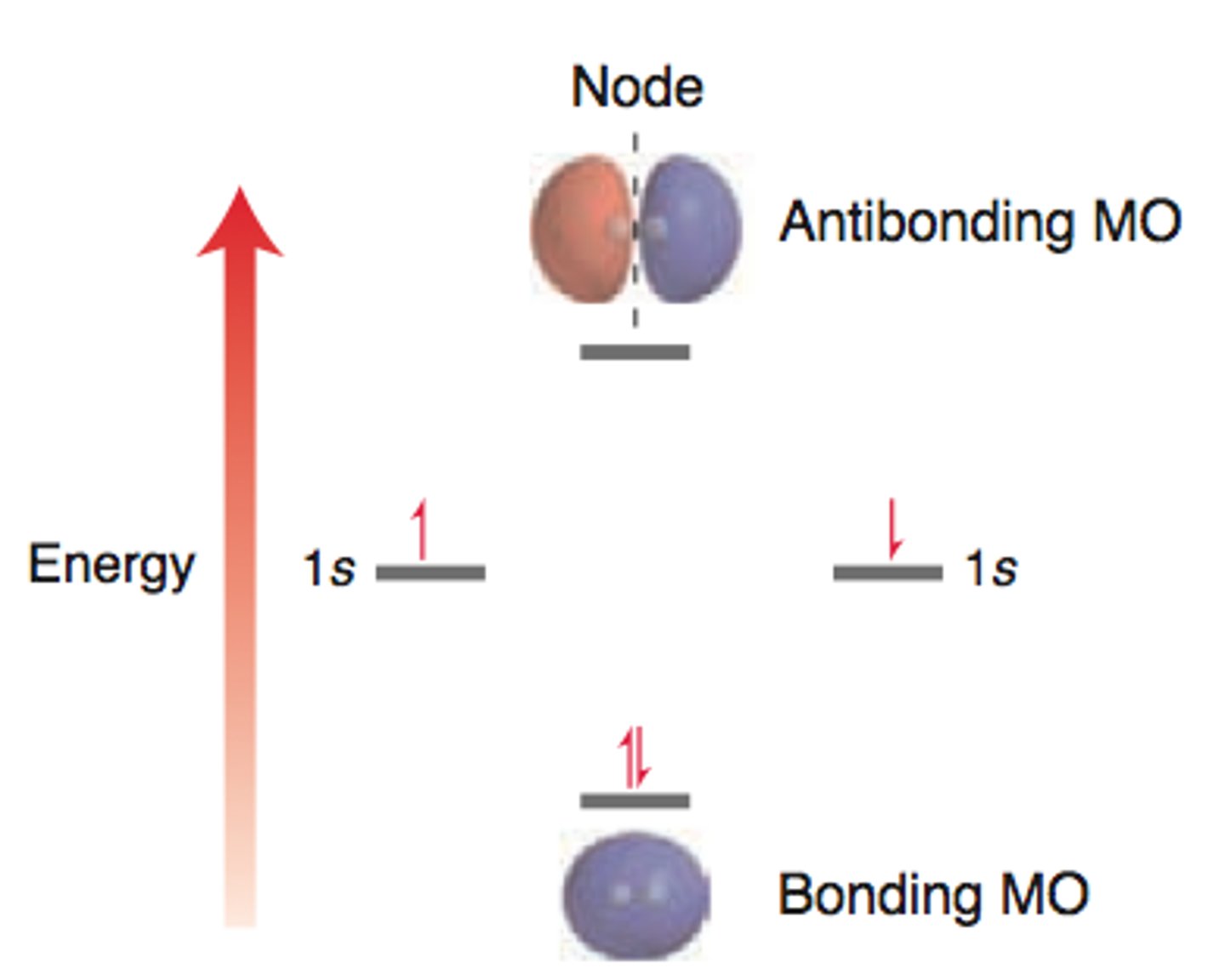
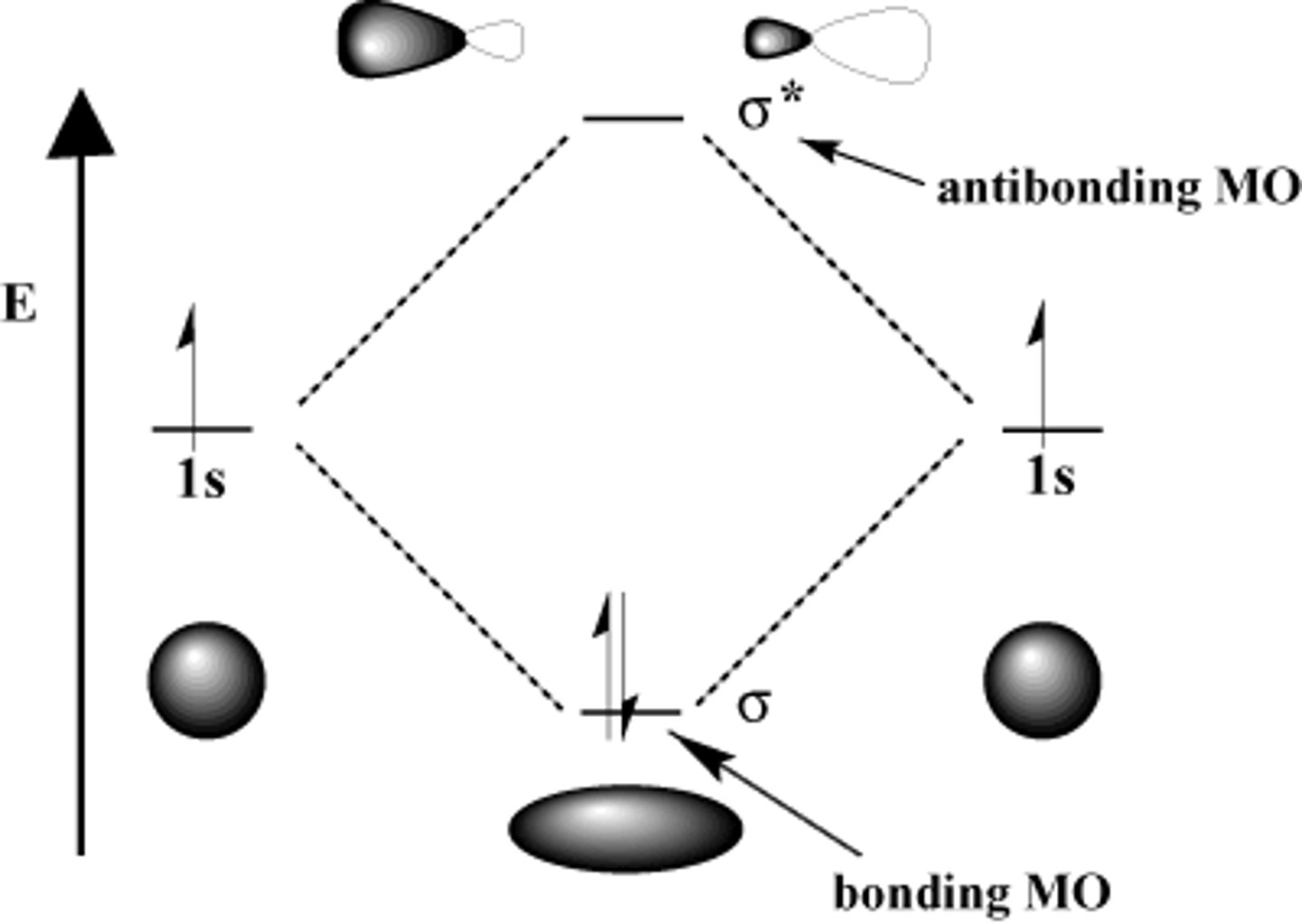
bonding orbital (𝜎)
high electron density between nuclei
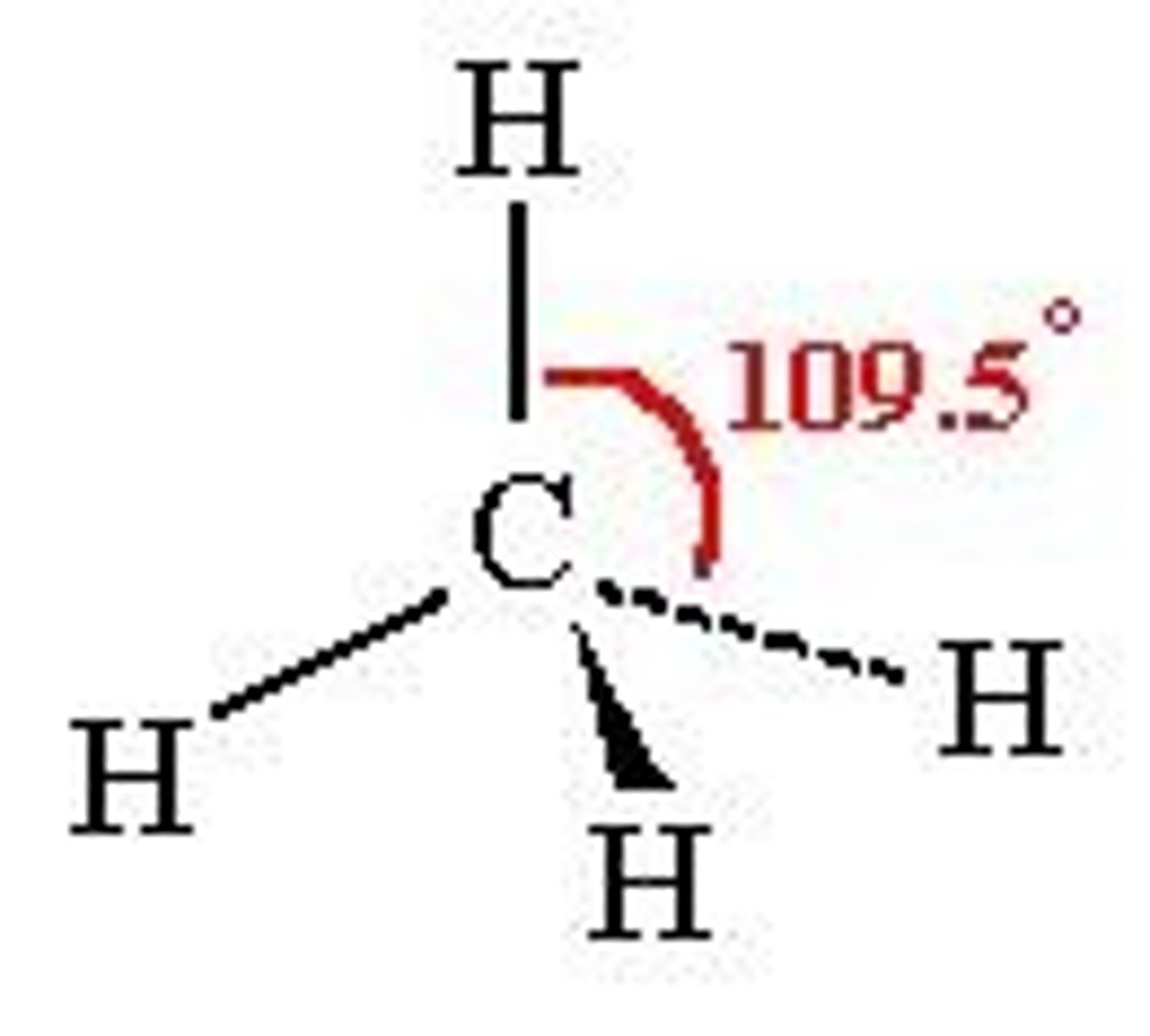
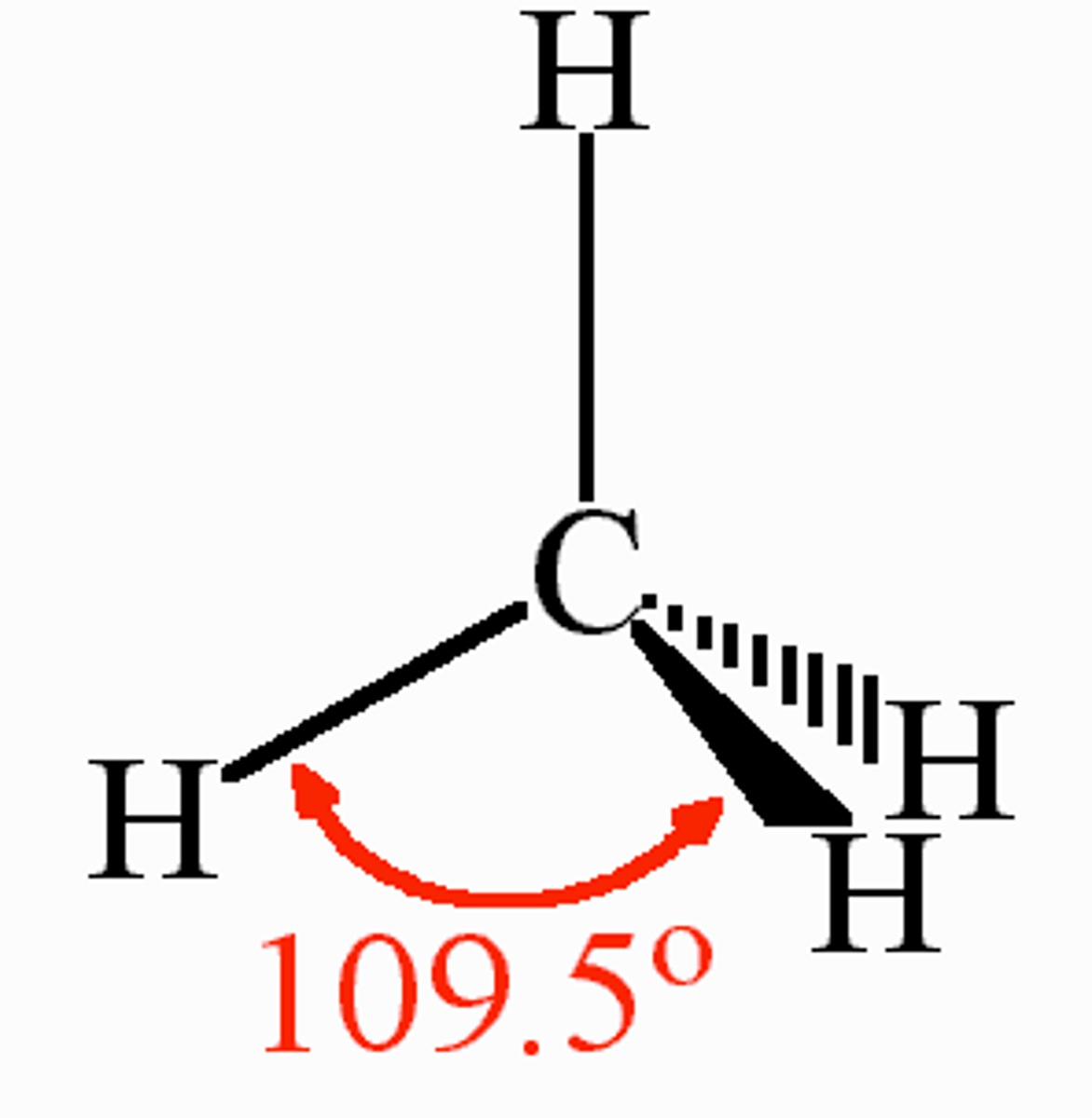
sp3 hybridization
tetrahedral, trigonal pyramidal, or bent
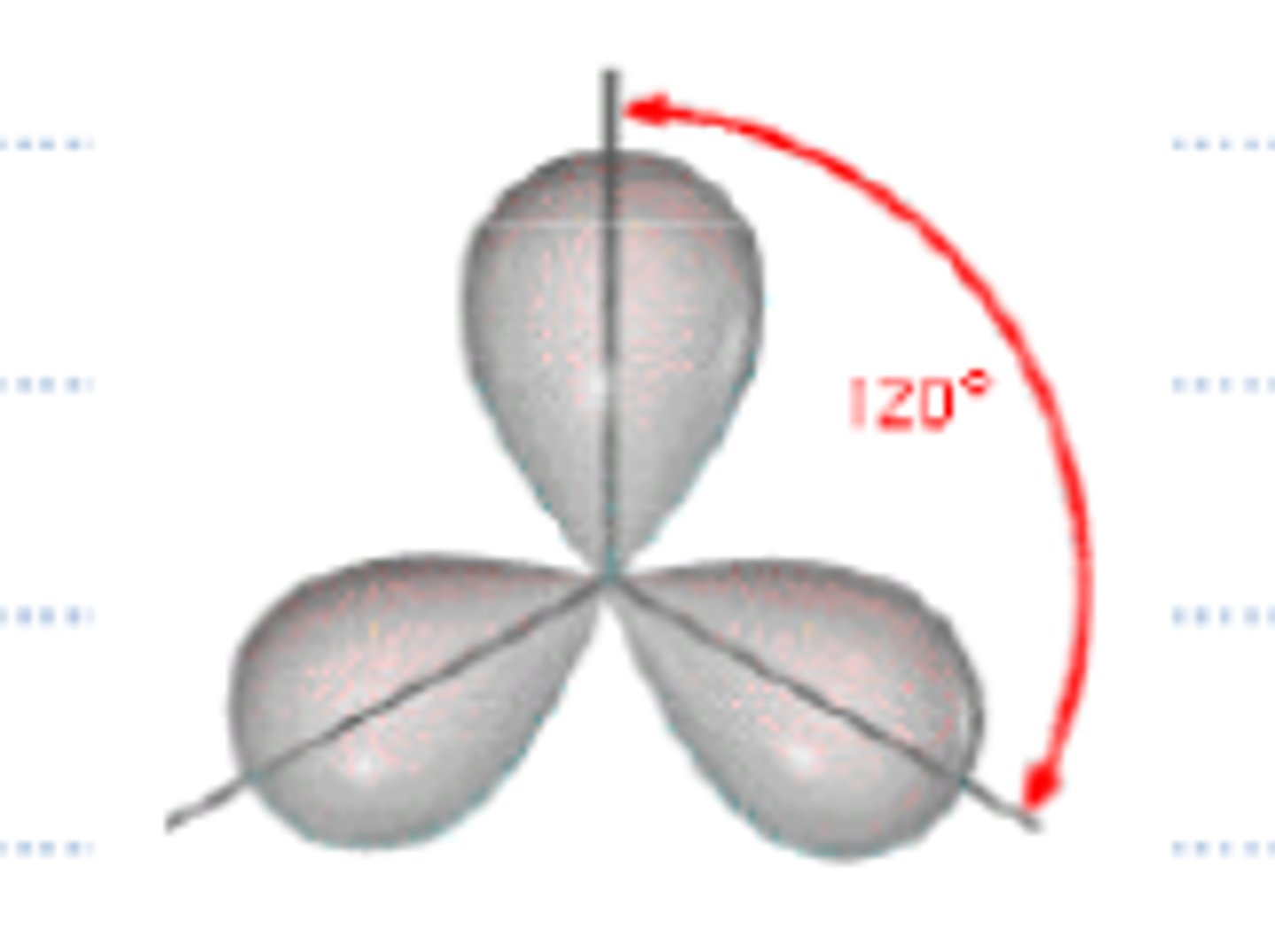
sp2 hybridization
trigonal planar
sp hybridization
linear
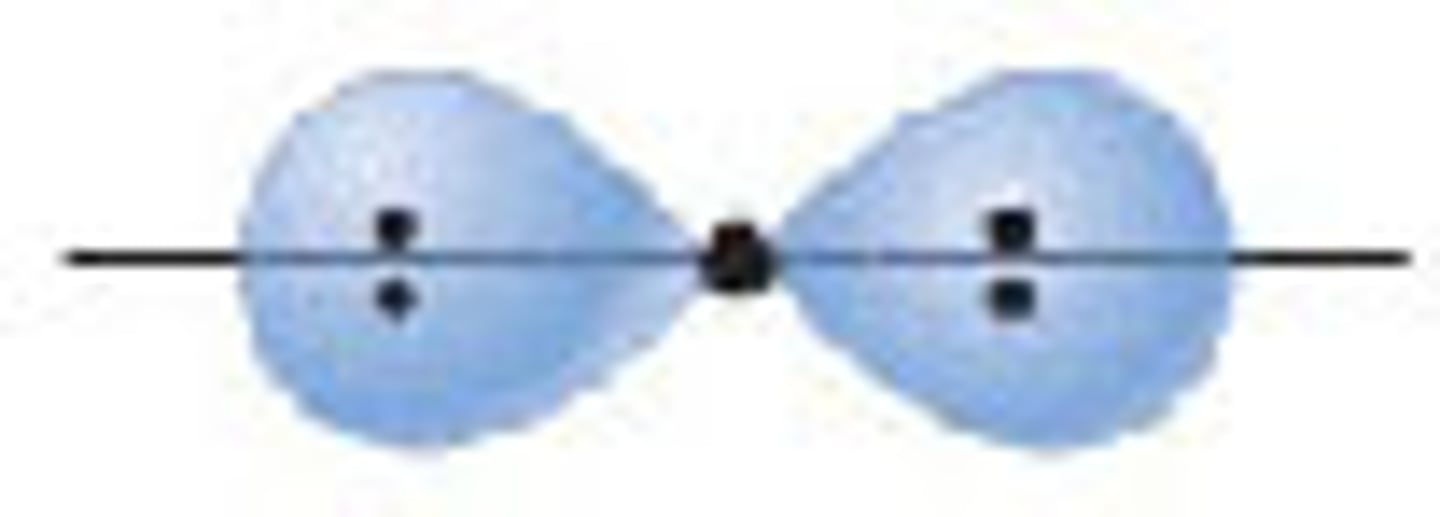
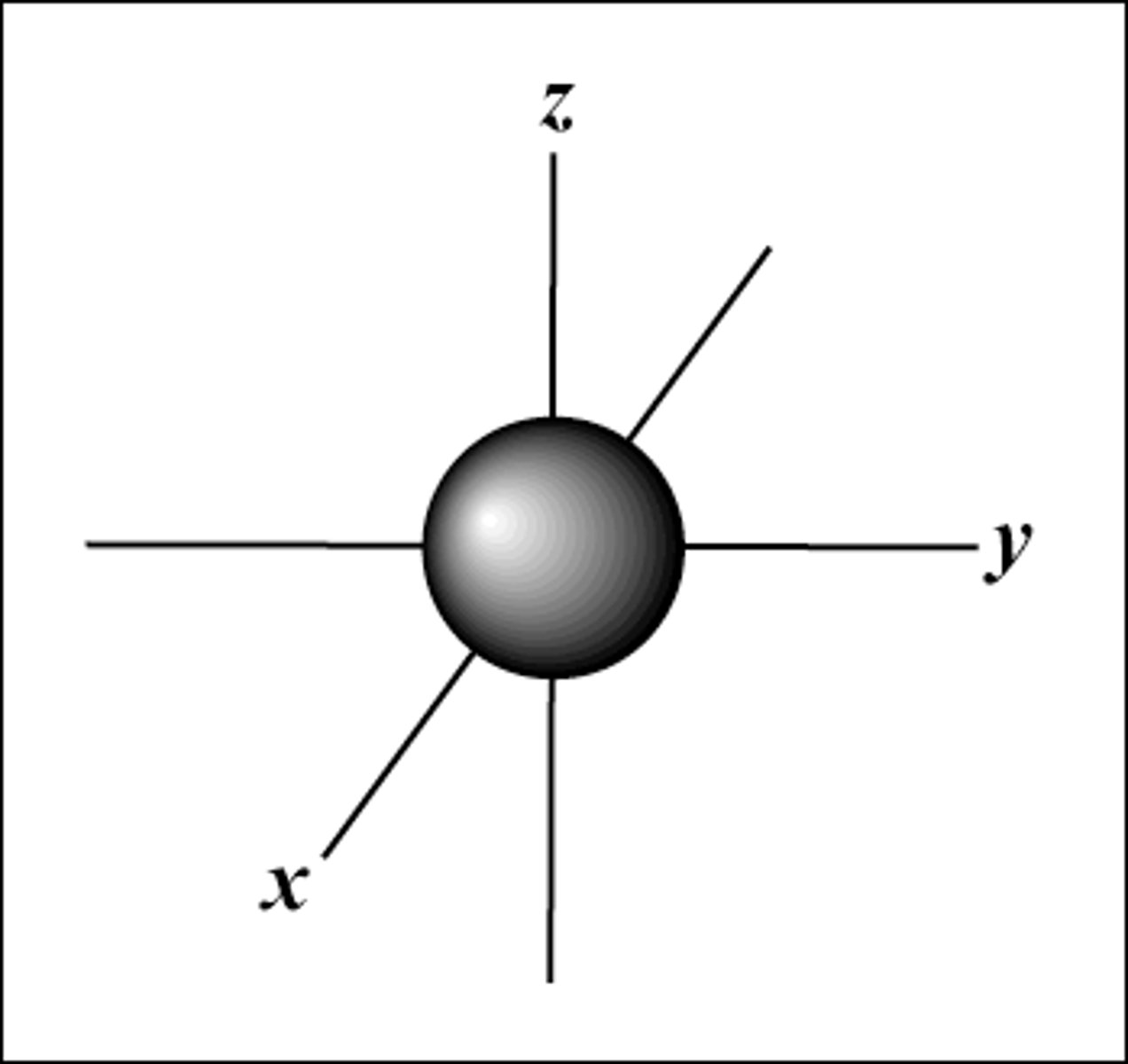
s orbital
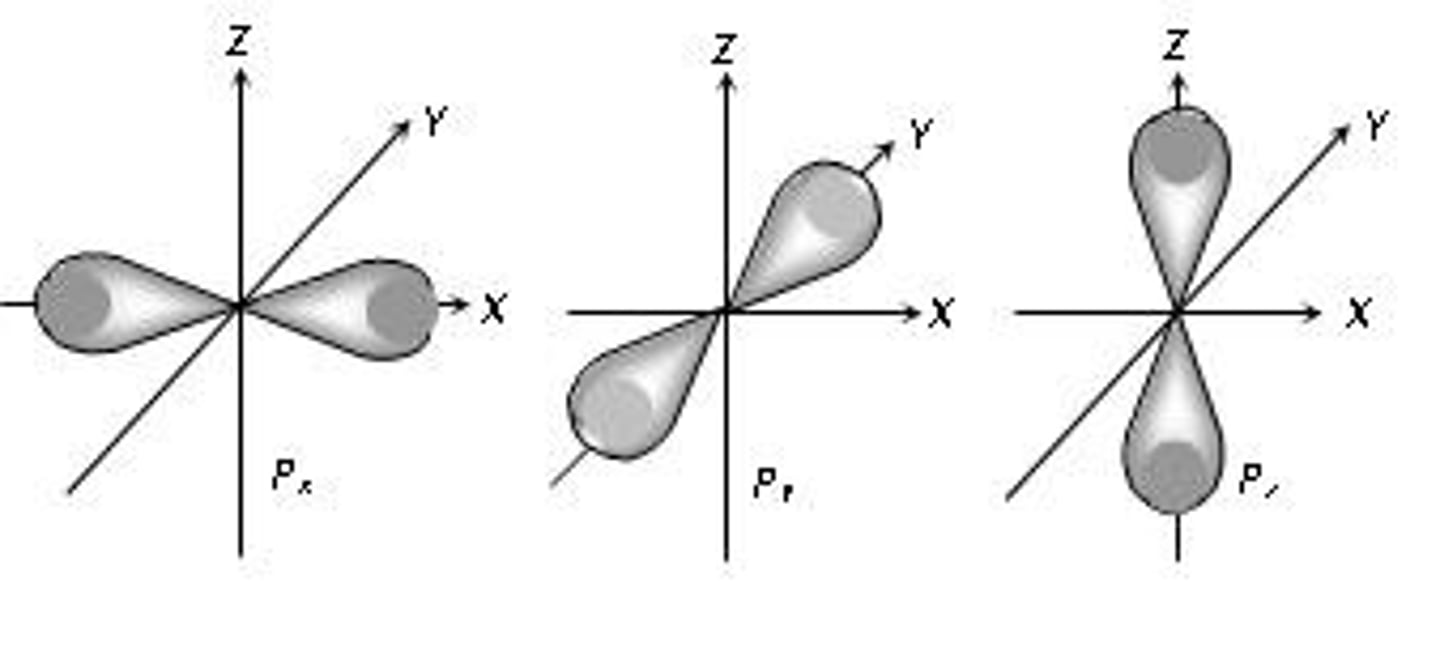
p orbital
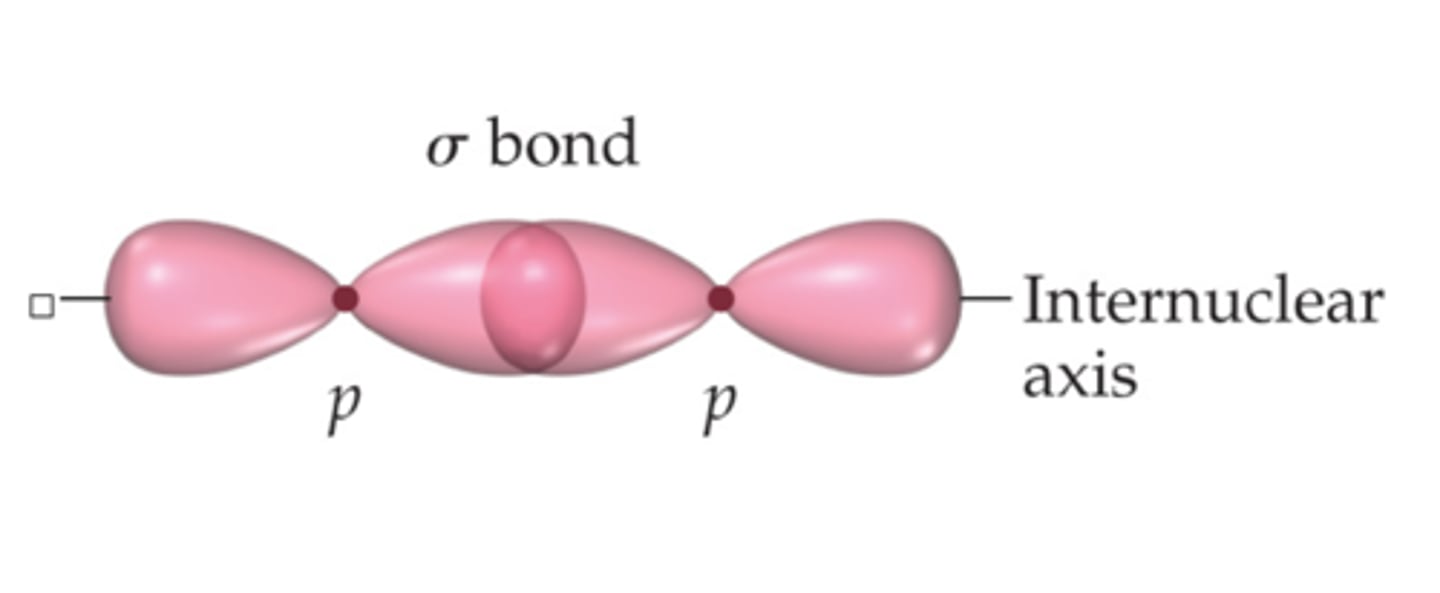
sigma bond
a bond formed when two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting the two atomic nuclei (there is always one sigma bond between two bonded atoms)
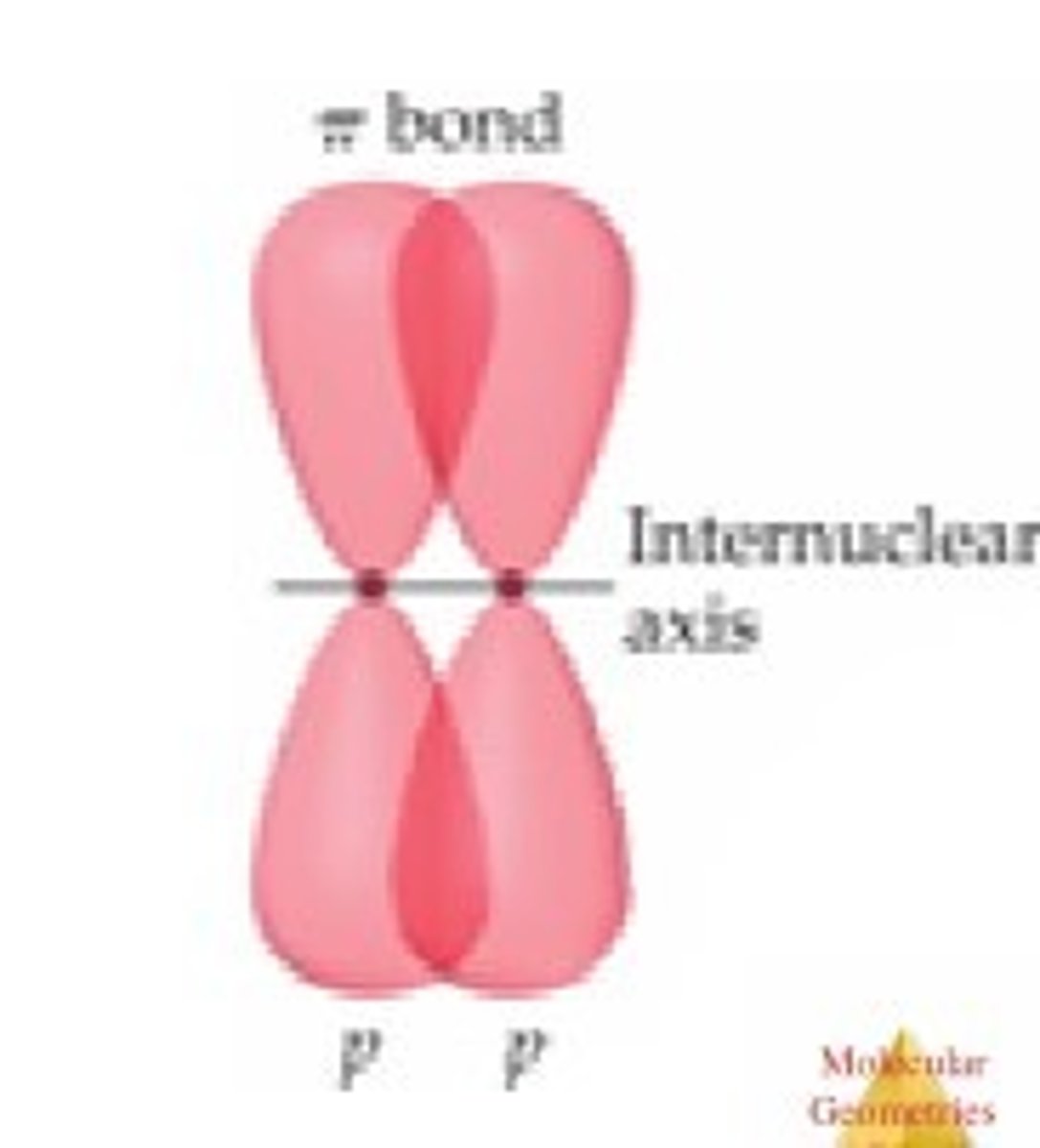
pi bond
a bond that is formed when parallel orbitals overlap to share electrons (any additional bond after a sigma!)
resonance structures
1. exist only on paper- the actual structures are hybrids of all the resonance structures
2. differ only in the position of electron pairs, not atoms
3. all structures should be proper lewis structures (a few exceptions)
4. all should have same number of unpaired electrons
hydrocarbons
compounds containing only hydrogen and carbon: alkanes, alkenes, and alkynes
generally not very reactive
isomer
compounds with the same molecular formula that differ in the order in which the atoms are bonded to one another; different chemical compounds with different chemical properties
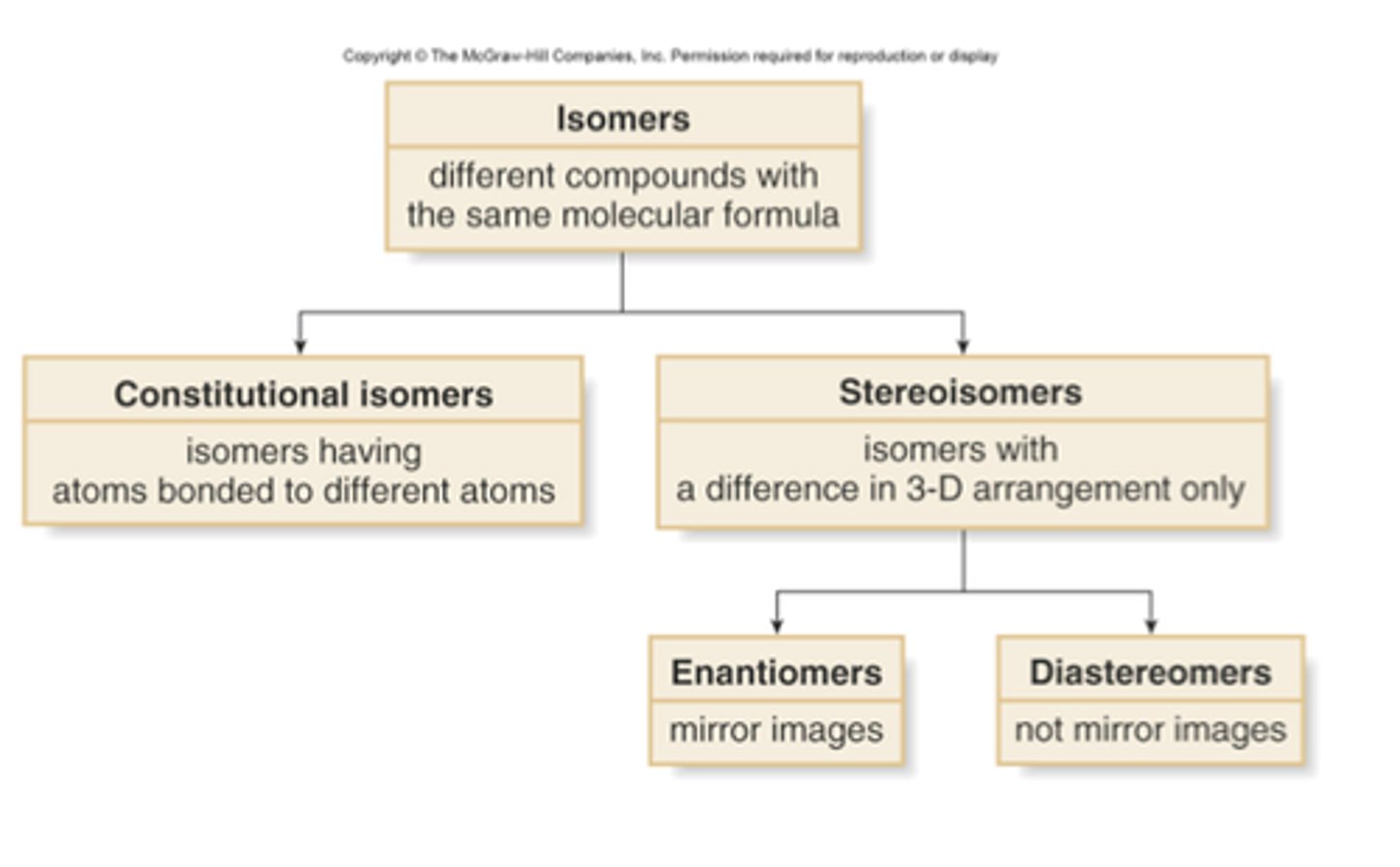
physical properties of alkanes
1. nonpolar compounds
2. branching lowers the boiling point due to the disruption of LDFs (branching as in up/down connections vs a straight chain)
3. insoluble in water; soluble in organic solvents like diethyl ether, benzene
4. very unreactive
5. more carbons = higher BP
constitutional isomers
compounds having the same numbers of each type of atom but with a different order of attachment
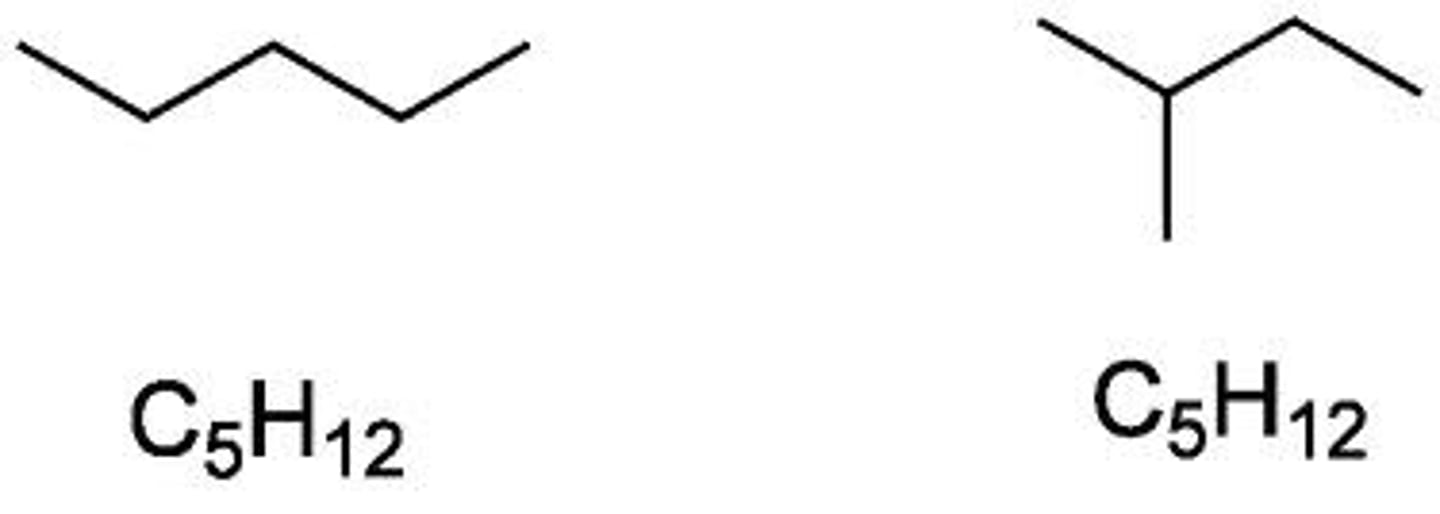
properties of constitutional isomers
1. BPs and MPs increase with increasing size
2. branching decreases BPs and MPs but increases stability
Why do alcohols have higher boiling points than ethers?
Alcohols can H-bond, ethers can only have dipole-dipole interactions
complete combustion
A combustion reaction in which the only products are carbon dioxide and water
incomplete combustion
Combustion in which not enough oxygen is supplied to completely burn the fuel. Carbon monoxide is a common product or solid carbon (soot)
heat of combustion
energy released when a compound is completely oxidized to carbon dioxide and water; depends mostly on number of CH2 units
a lower heat of combustion means that the product is more stable: BRANCHED compounds tend to be more STABLE than their unbranched counterparts
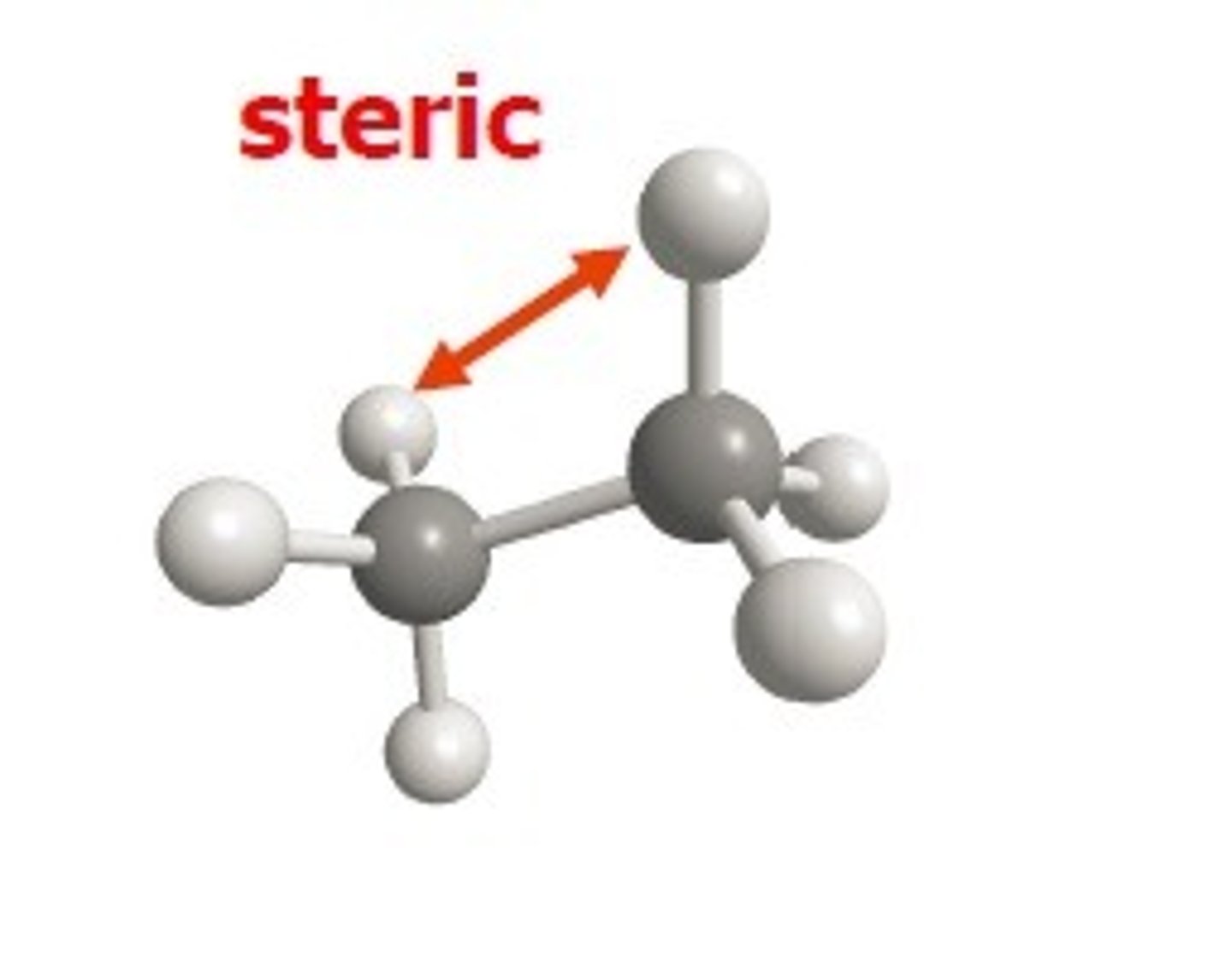
steric energy
isolated molecule in gas phase at 0 K, relative energy of a conformation or stereoisomer calculated using classical mechanics
stretch
bond length; energy associated with stretching or compressing bonds from their optimal length
bend
bond angle; energy associated with deforming bond angles from their optimal angle
stretch-bend
energy required to stretch two bonds involved in a severly compressed bond angle
dipole-dipole
energy associated with interaction of bond dipoles
out of plane
energy required to distort a trigonal center out of planarity
torsional strain
destabilization from eclipsing of bonds on adjacent atoms
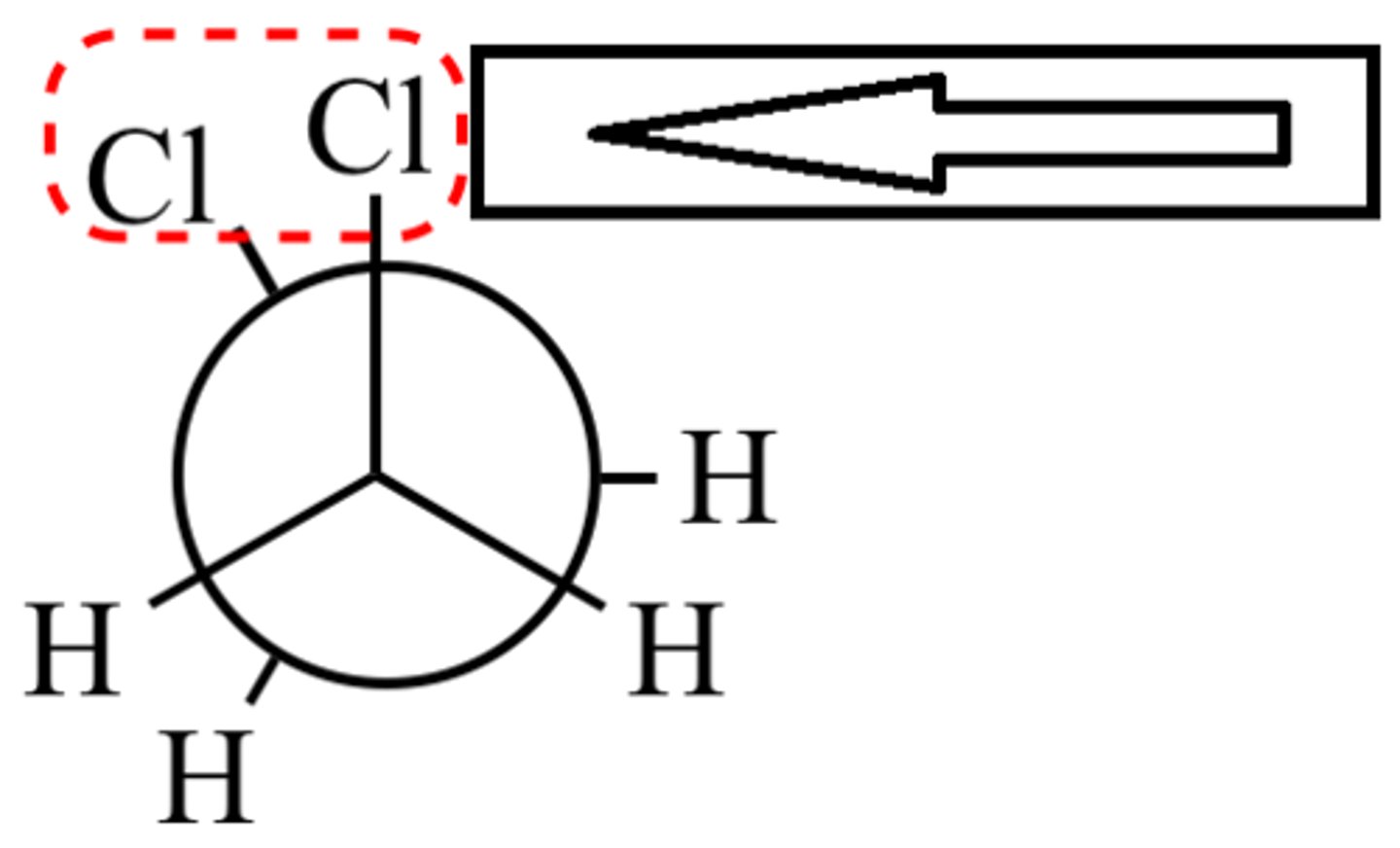
van der waals strain
destabilization from two atoms being too close together
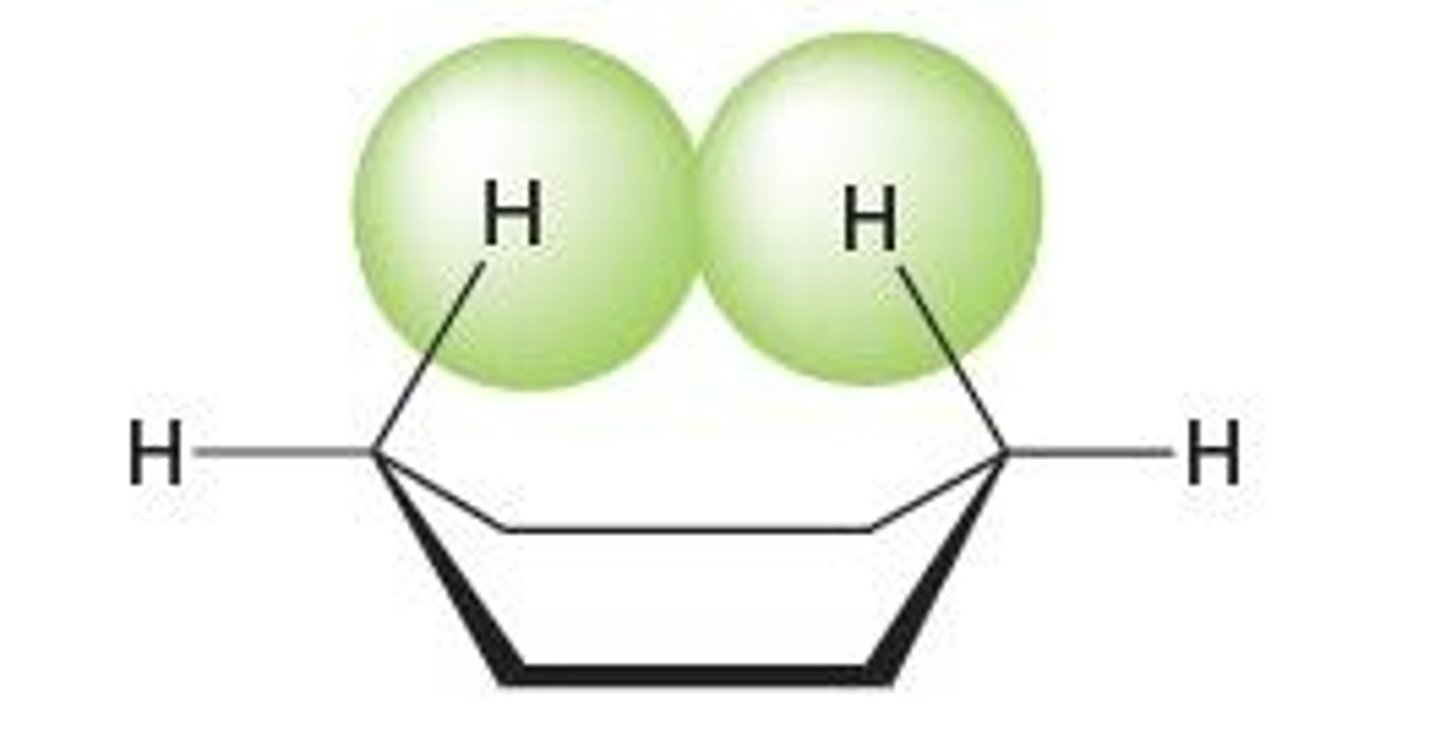
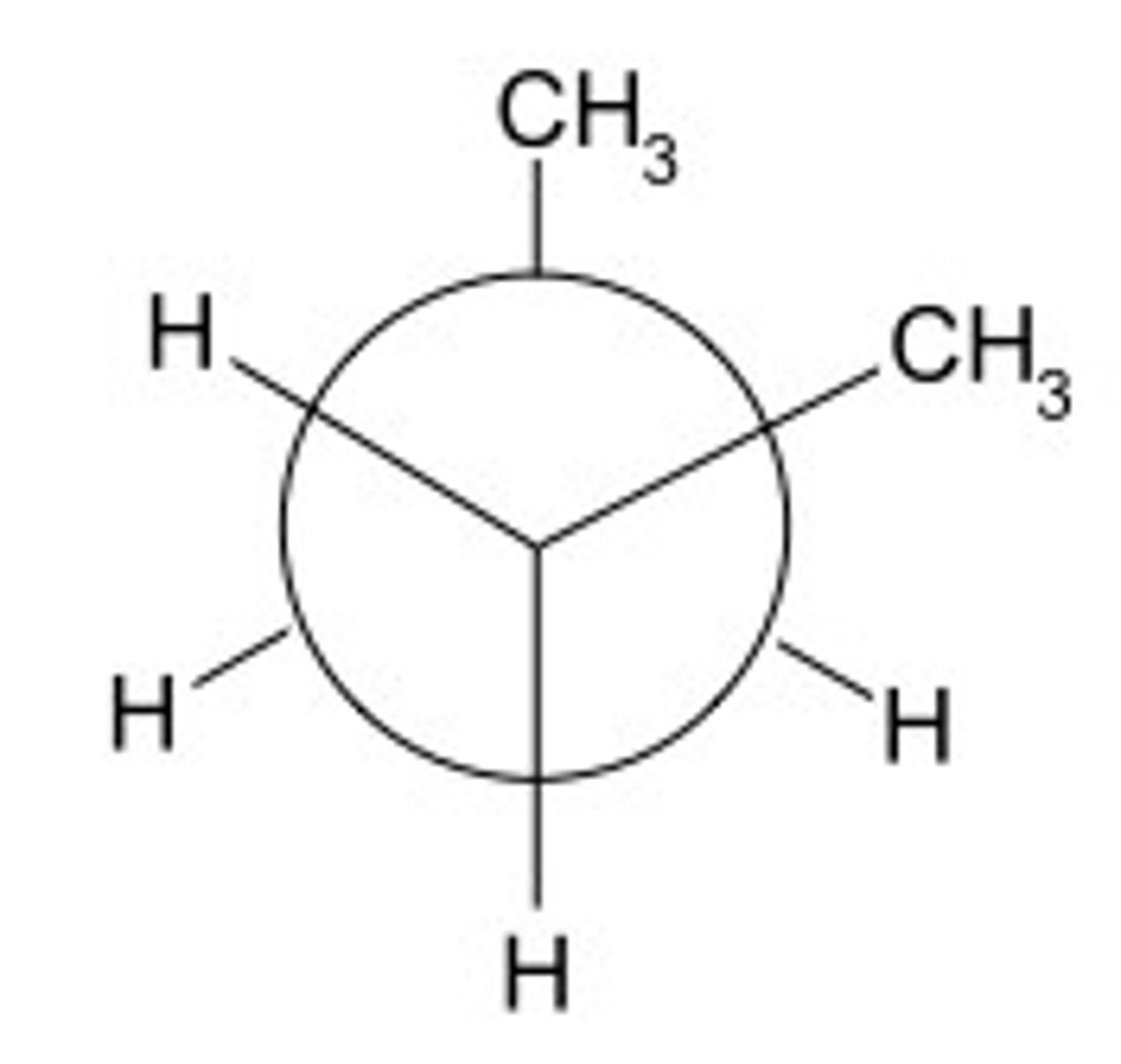
dihedral angle
the angle between two specified groups in a Newman projection
dimensional projection
shows molecules in 3D
Newman projection
A method of visualizing a compound in which the line of sight is down a carbon-carbon bond axis.
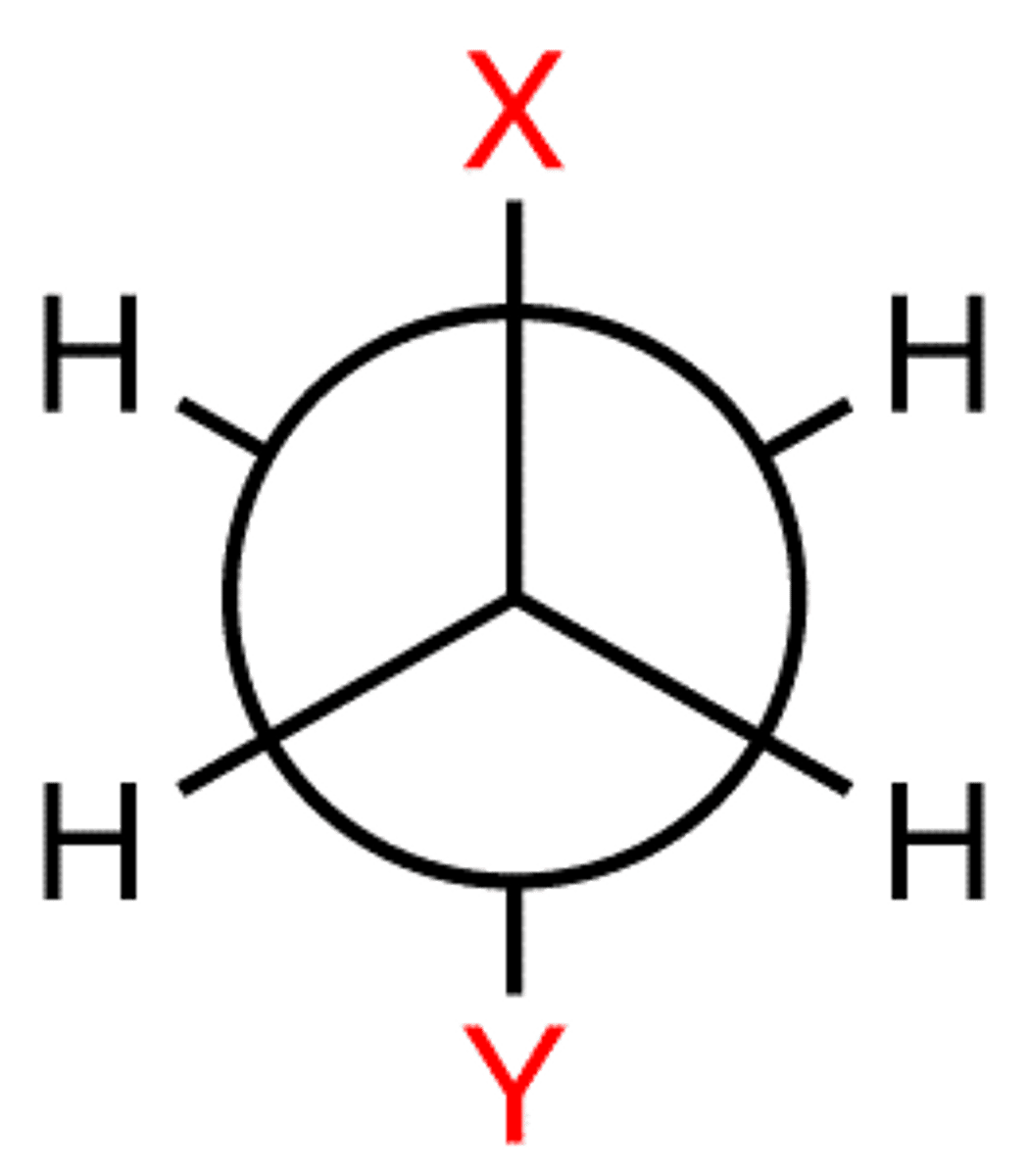
staggered
a chemical conformation of an ethane-like molecule in which the substituents are at the maximum distance from each other
dihedral angle is 60°
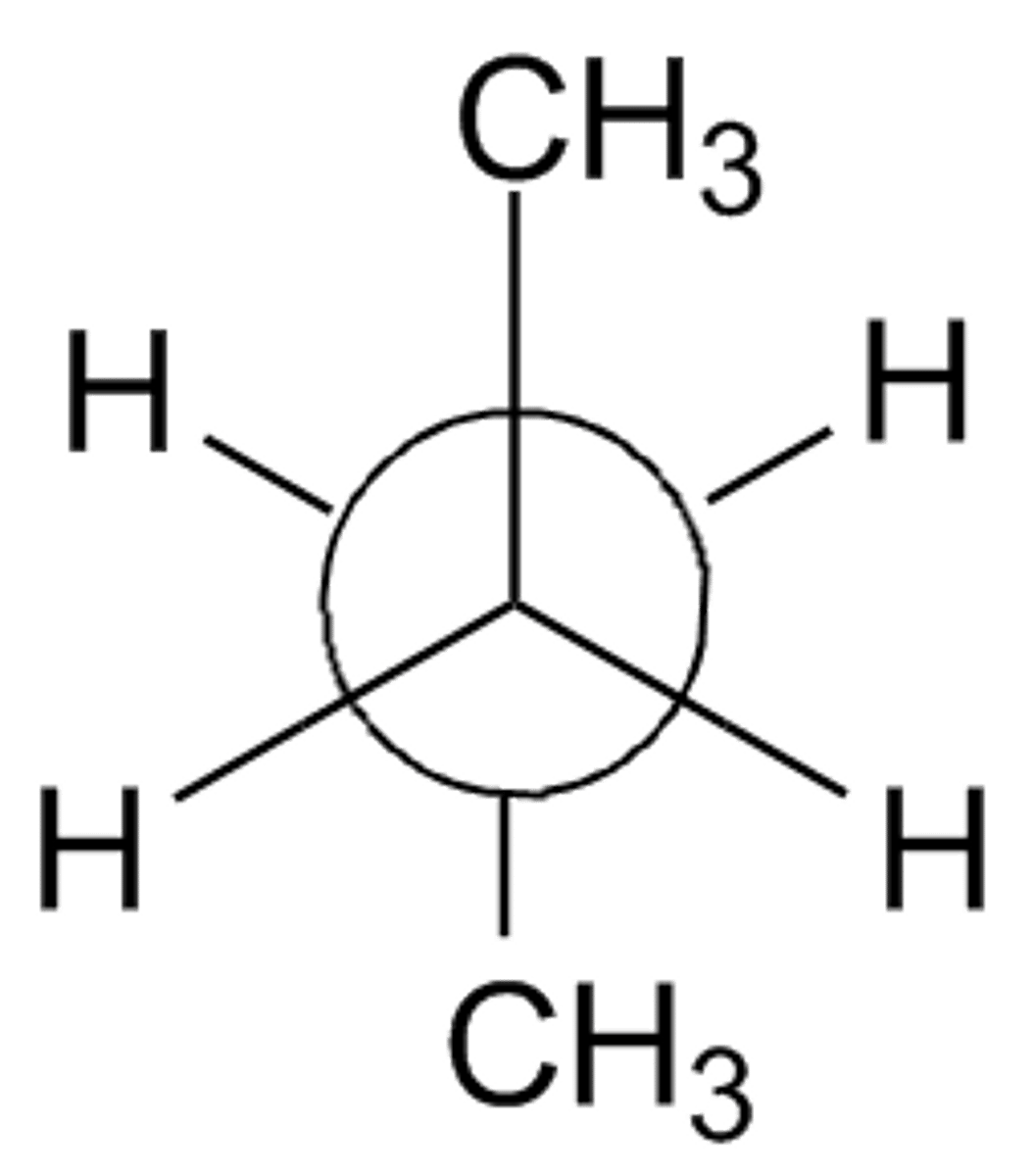
eclipsed
two atoms/groups whose dihedral angle is zero, least stable configuration; highest in energy
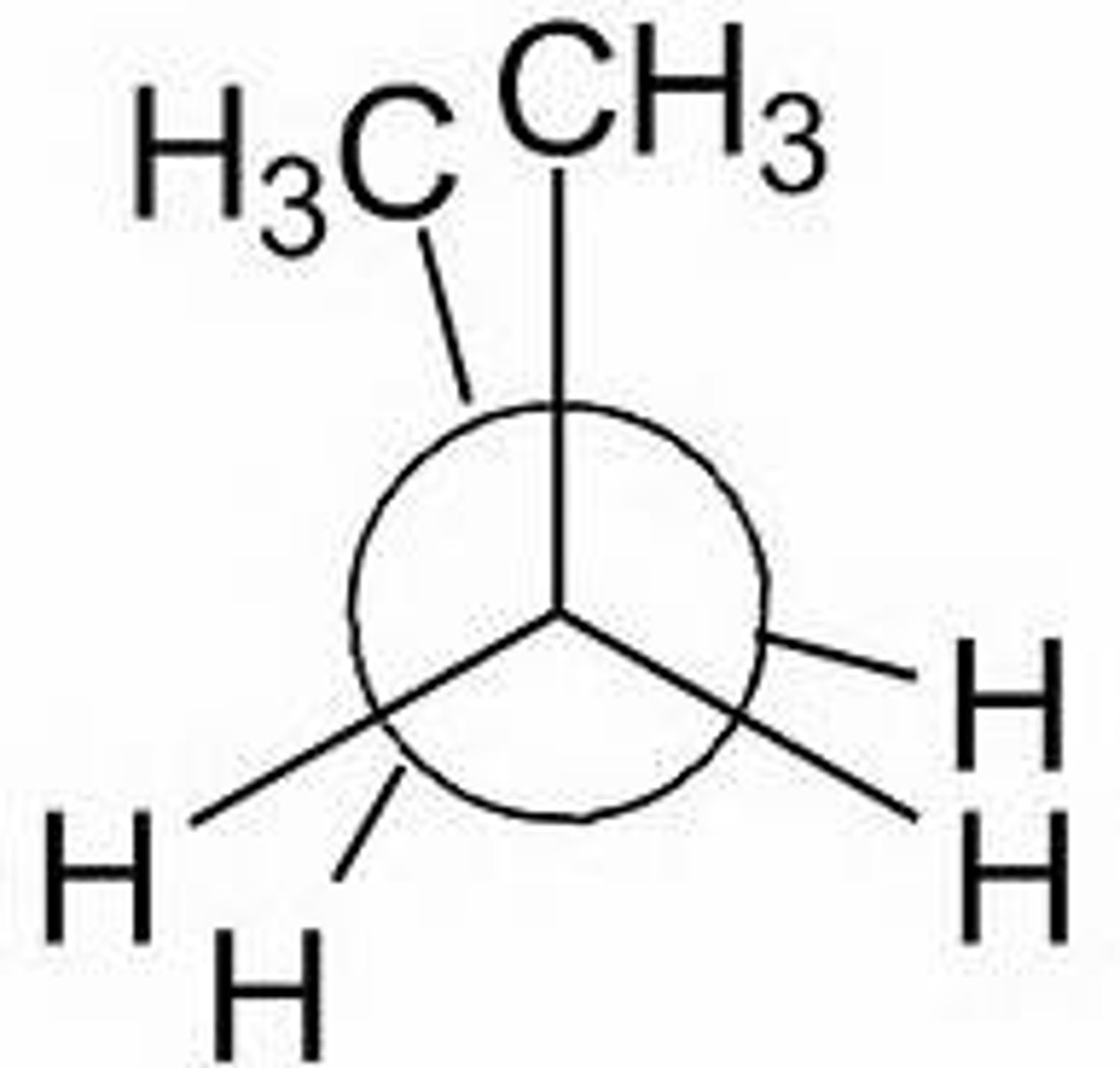
anti
atoms/groups point in opposite directions, 180 dihedral angle
gauche
molecules (also usually of the same type) being 60° from each other on a Newman projection
ring strain
as the ring gets bigger, the strain energy goes down
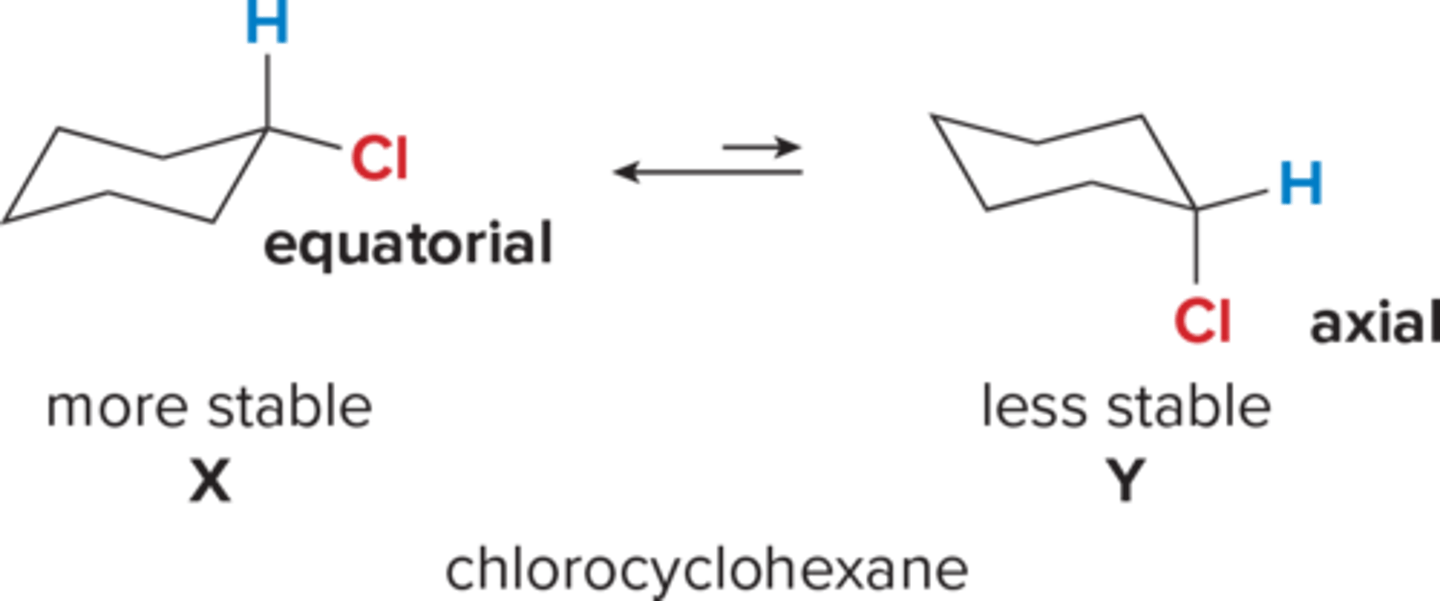
equatorial bonds
Bonds on cyclohexane chair parallel to the ring
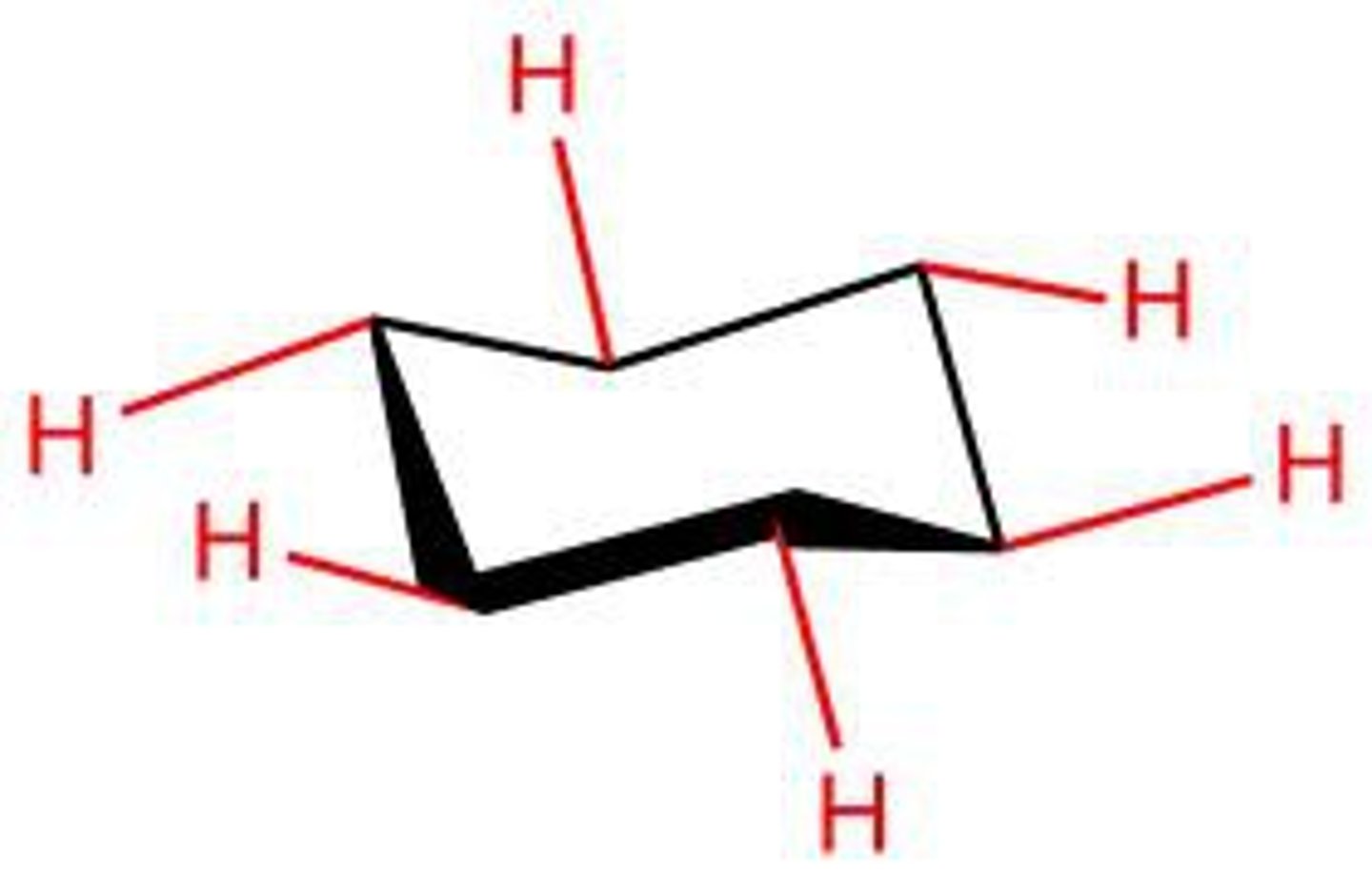
axial bonds
vertical and alternate above and below the ring
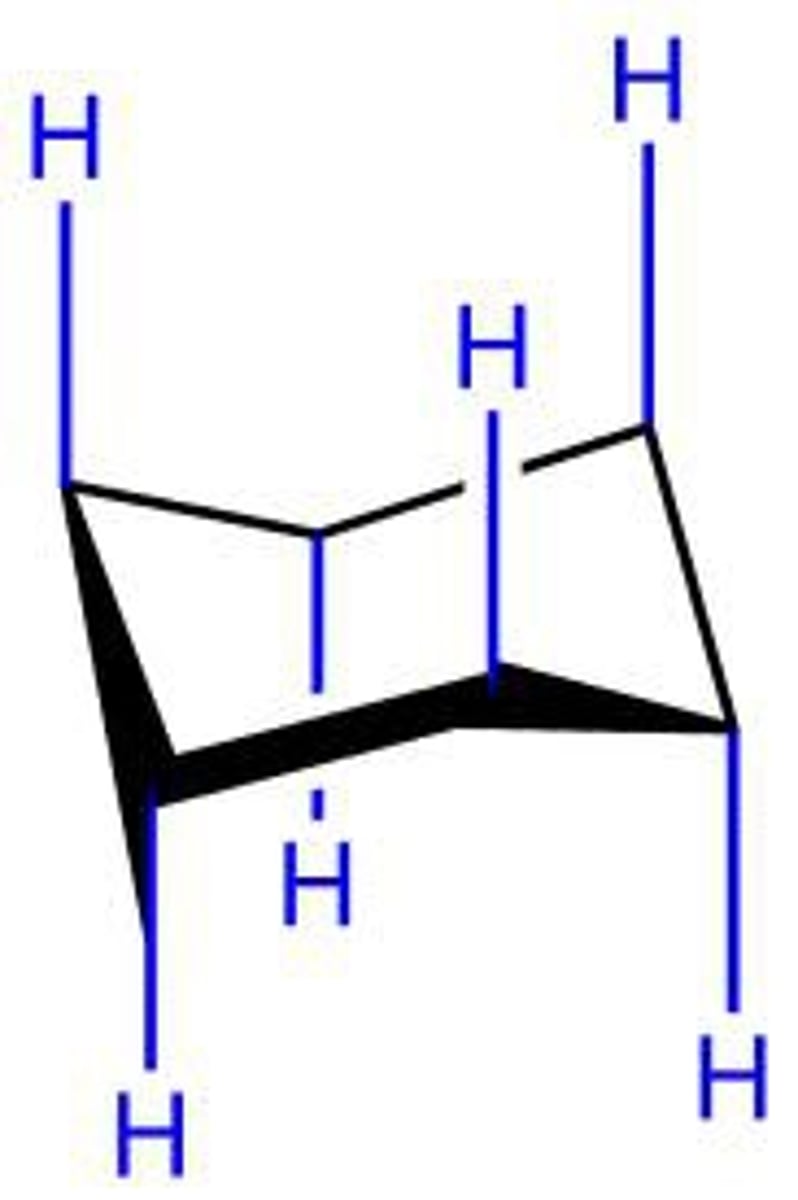
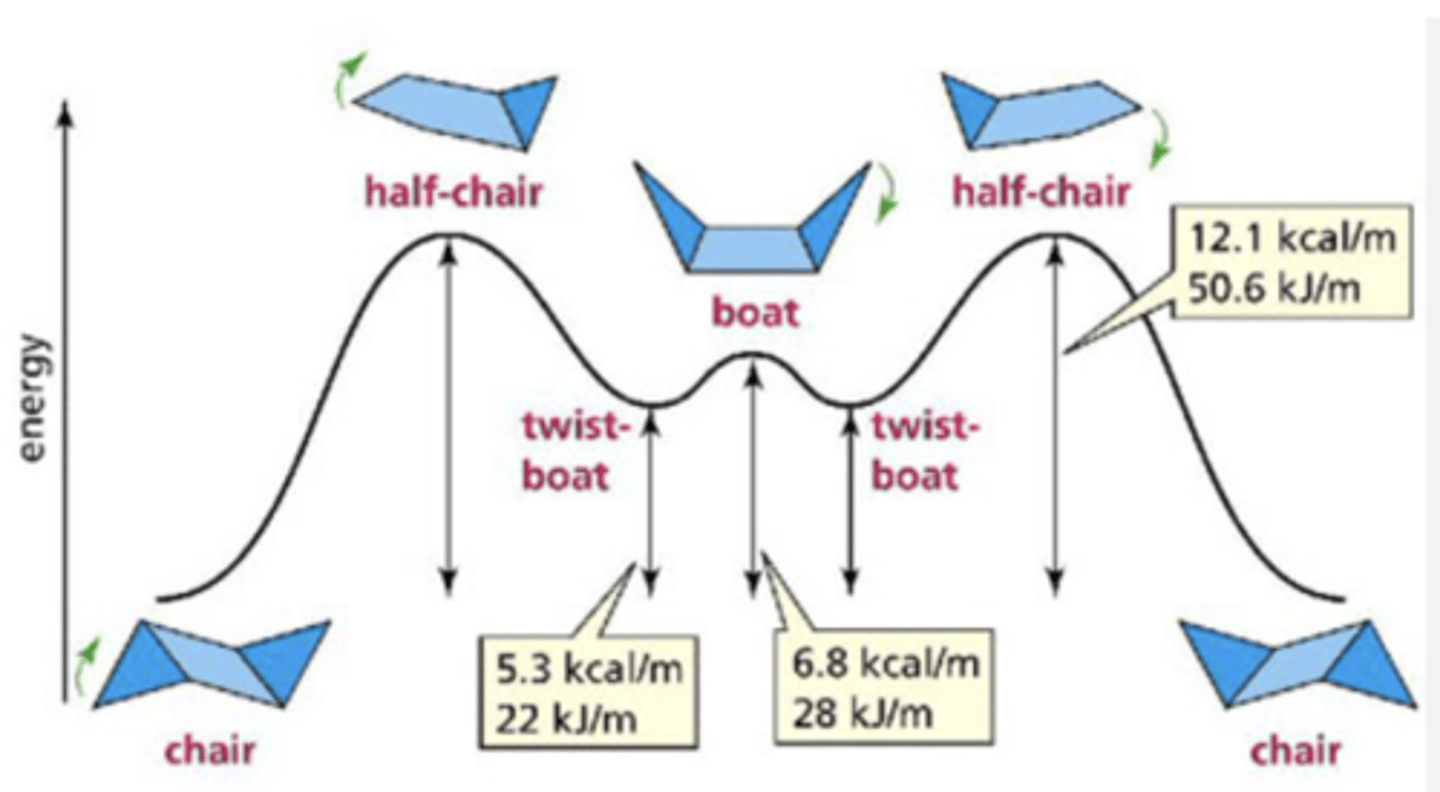
chair conformation of cyclohexane
most stable conformation of cyclohexane, lowest energy
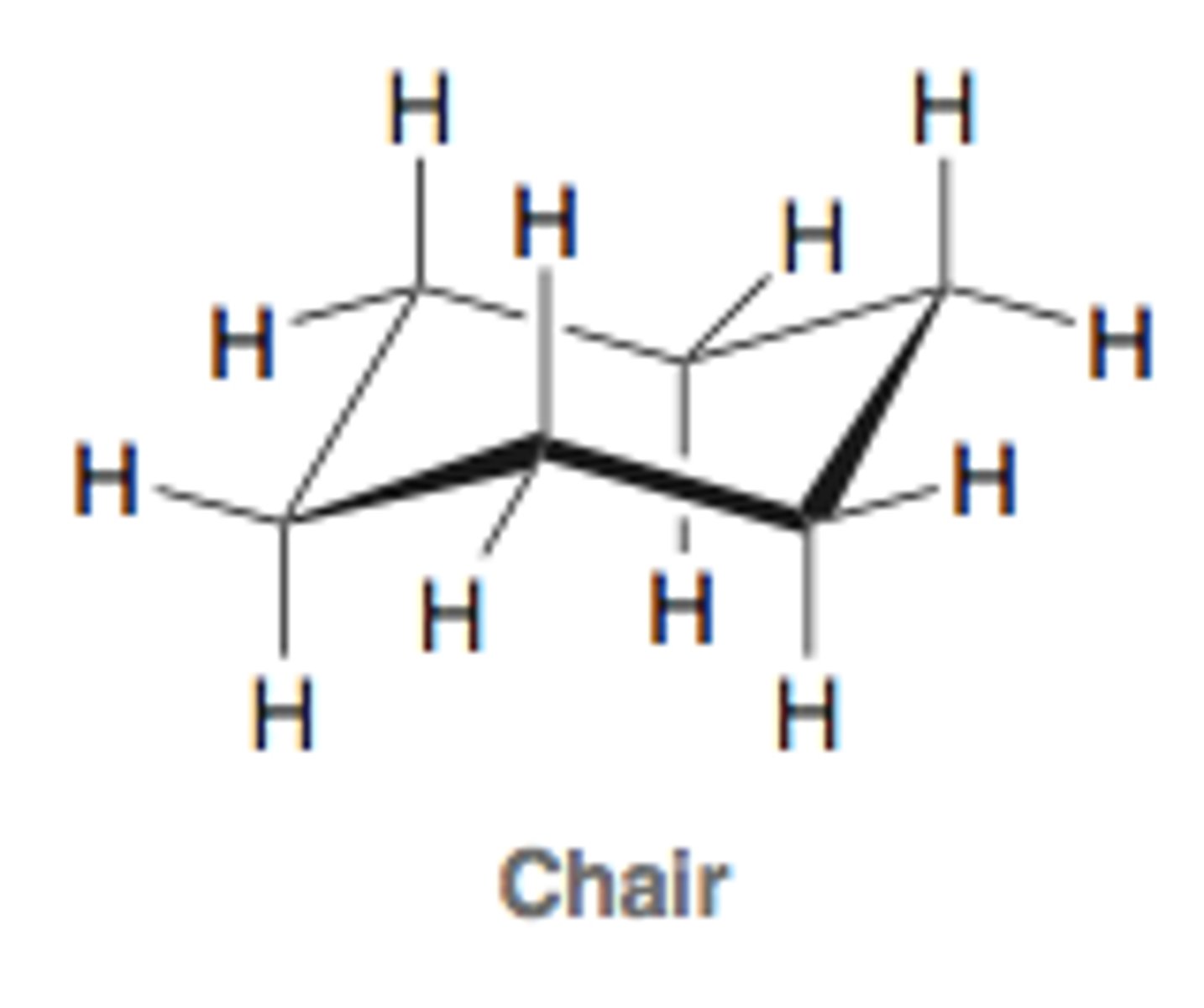
half-chair conformation of cyclohexane
the unstable conformation halfway between the chair conformation and the boat conformation; highest energy
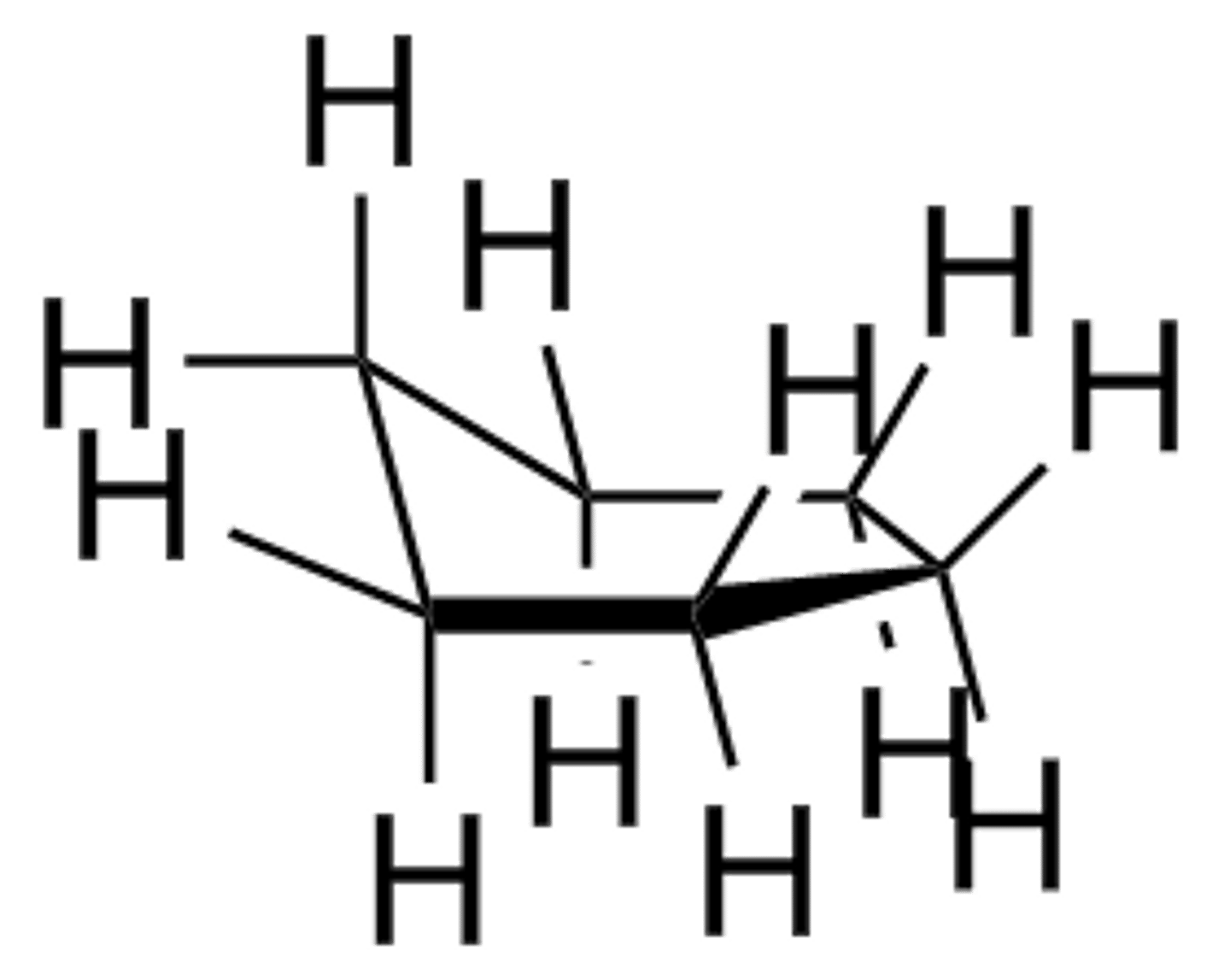
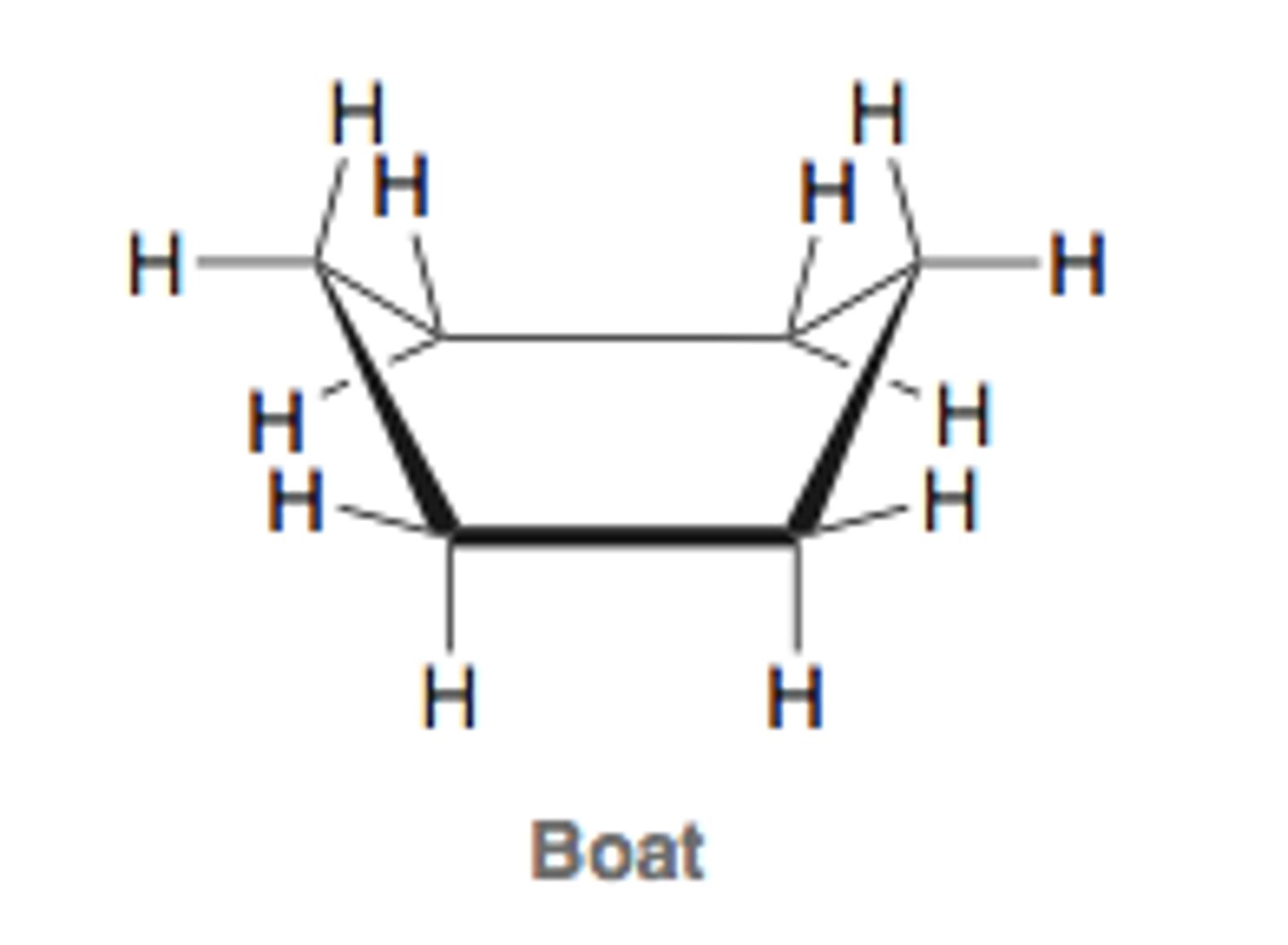
boat conformation of cyclohexane
least stable conformation of cyclohexane
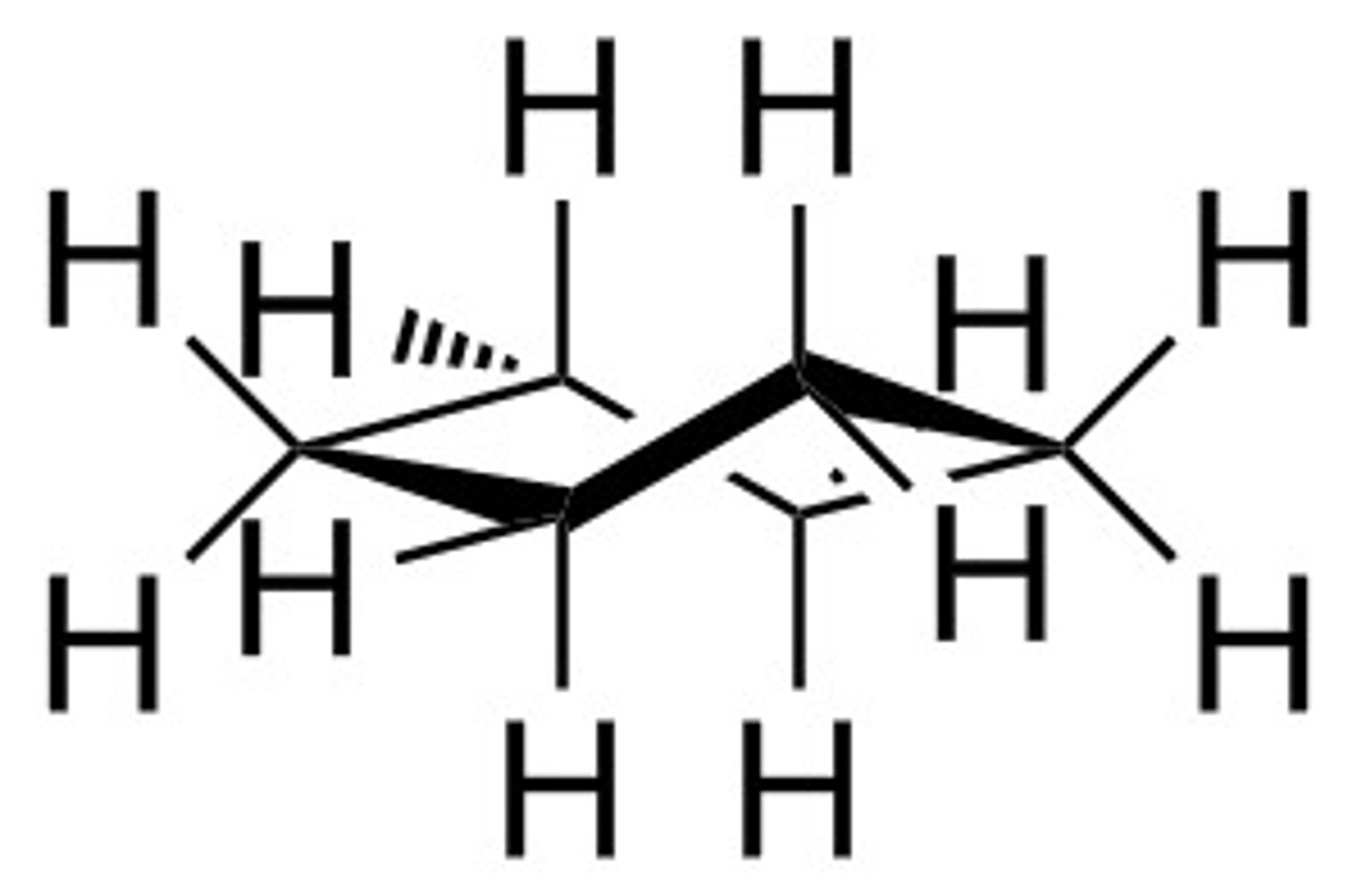
twist boat
more stable than boat, less stable than chair, less stable than a half chair
conformations of cyclohexane
substituted cyclohexanes
preferentially occupy equatorial positions due to 1,3 diaxial interactions in axially substituted cyclohexanes
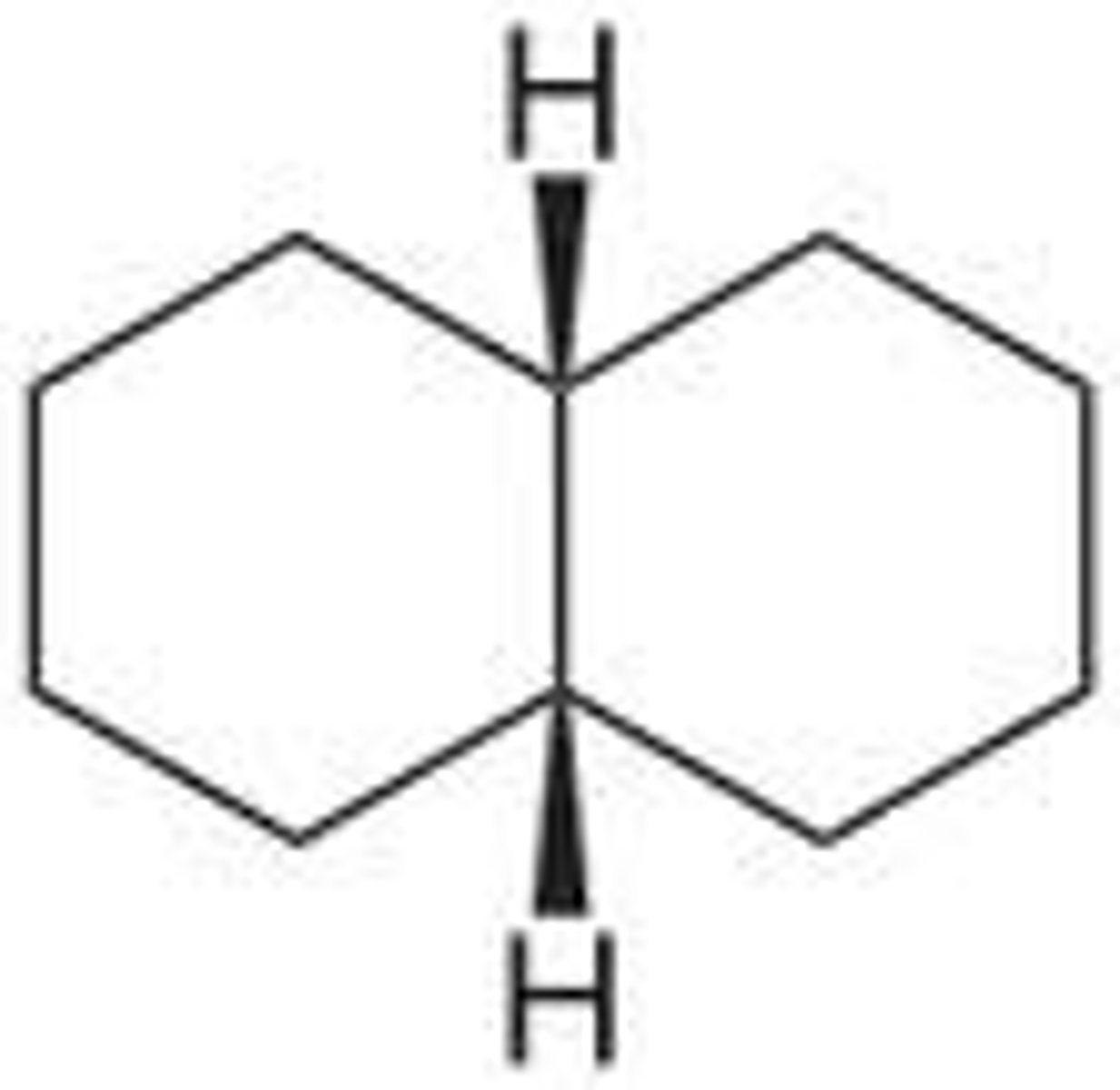
bicyclic compounds
compounds that contain two fused rings, conformationally "locked," chairs cannot flip back and forth between conformations, ex decalin
stereoisomers
compounds with the same structures differing only in their arrangement of atoms in space; not cis or trans
rules for assigning priority for stereoisomers (E/Z notation)
1. if the atoms are different, highest atomic number gets highest priority
2. if there are two isotopes of the same element, the one with the higher mass gets the higher priority
3. if the atoms are the same, the atomic numbers of the next atoms are used to assign priority
4. atoms attached by double or triple bonds are given single bond equivalencies
(E)
across, opposite sides of the bond
(Z)
together, priority groups are on on same sides of the bond
chiral
an object or molecule which cannot be superimposed on its mirror image; makes enantiomers
achiral
an object or molecule can be superimposed on its mirror image
enantiomers
isomers which are nonsuperimposable mirror images
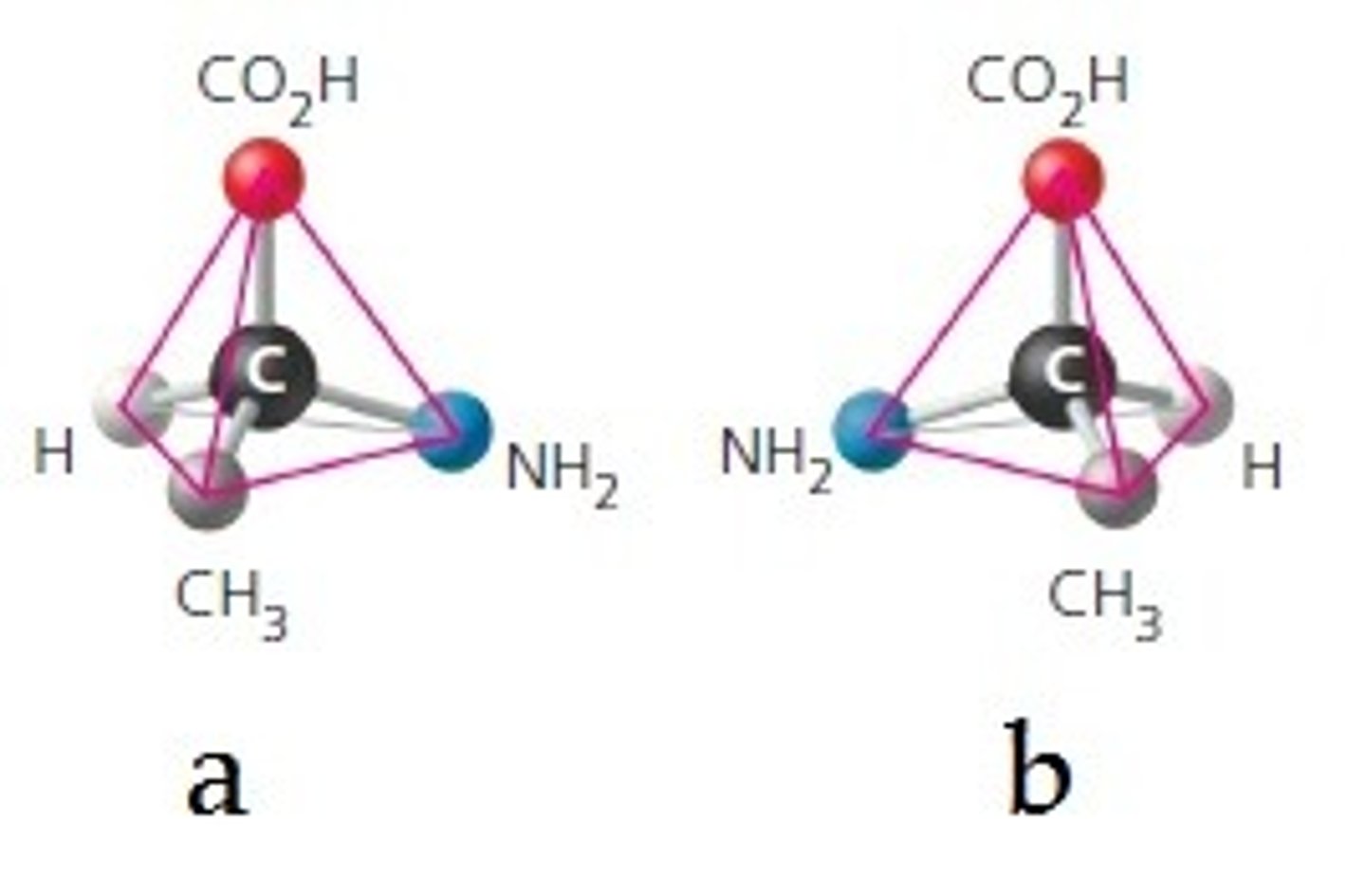
stereogenic carbon atom
also called asymmetric center or chiral center; the carbon atom with four different groups bonded to it
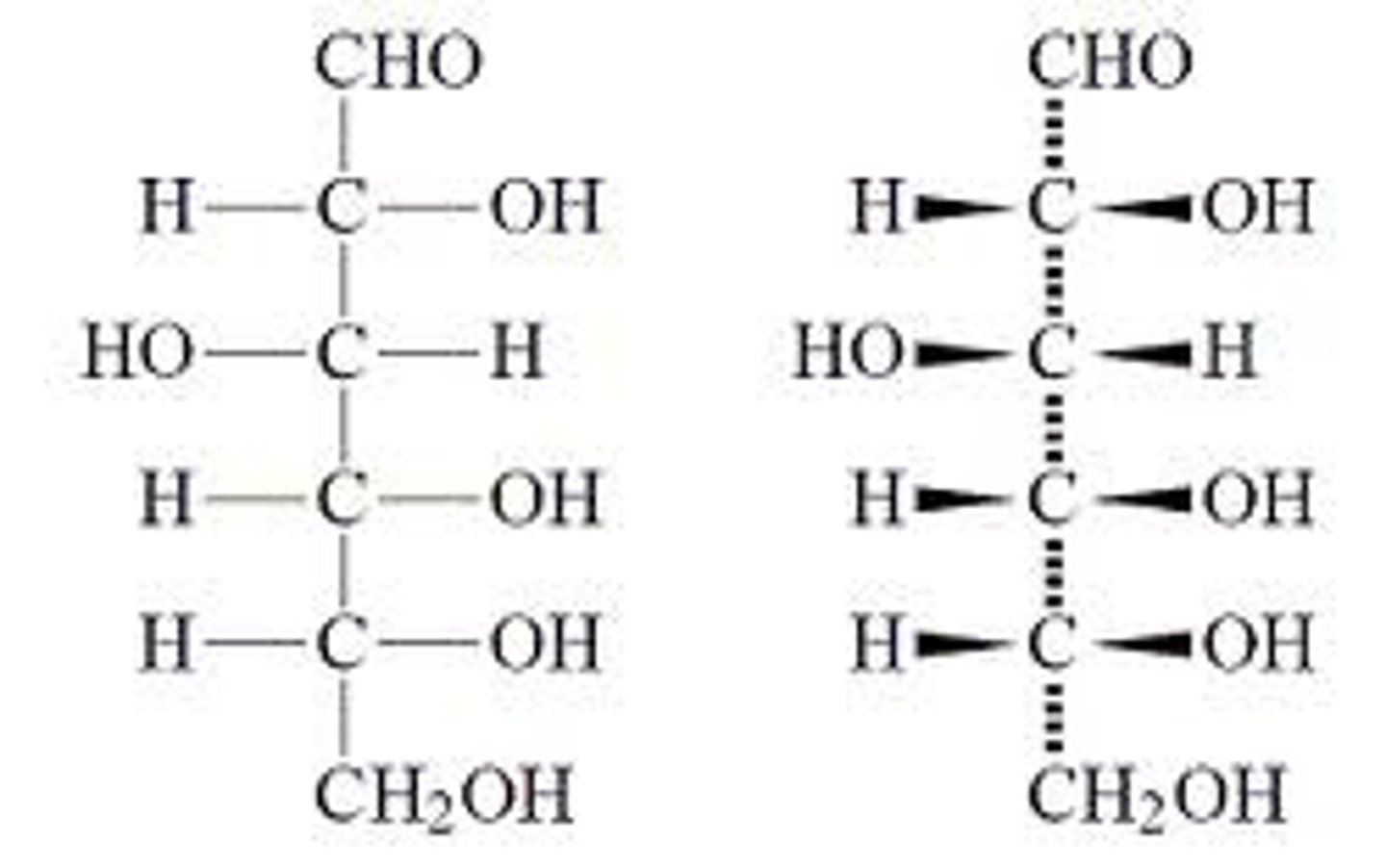
fischer projections
-Horizontal lines indicate bonds that project out from the plane of the page
-Vertical lines indicate bonds going into the plane of the page
-point of intersection = carbon atom
properties of enantiomers
have almost all of the same physical and chemical properties, what differs is...
1. the interaction with other chiral substances
2. interactions with polarized light
optically active
compound that rotates the plane of polarized light
optical isomers
enantiomers
racemic mixture
A mixture that contains equal amounts of the (+) and (-) enantiomers; does not rotate a plane of polarized light; therefore optically inactive
Lower MP than pure enantiomers (each enantiomer depresses the other); designated by (±)
R-S notational system
1. assign priorities to the substituents according to CIP priority rules
2. orient the molecule with the lowest group pointing away (rear)
3. turn the wheel 1, 2, 3
4. if the direction of the semicircle is clockwise, the configuration is R. if it is counterclockwise, the configuration is S.
*remember, sp3 carbons are only chiral if all of their bonds are different
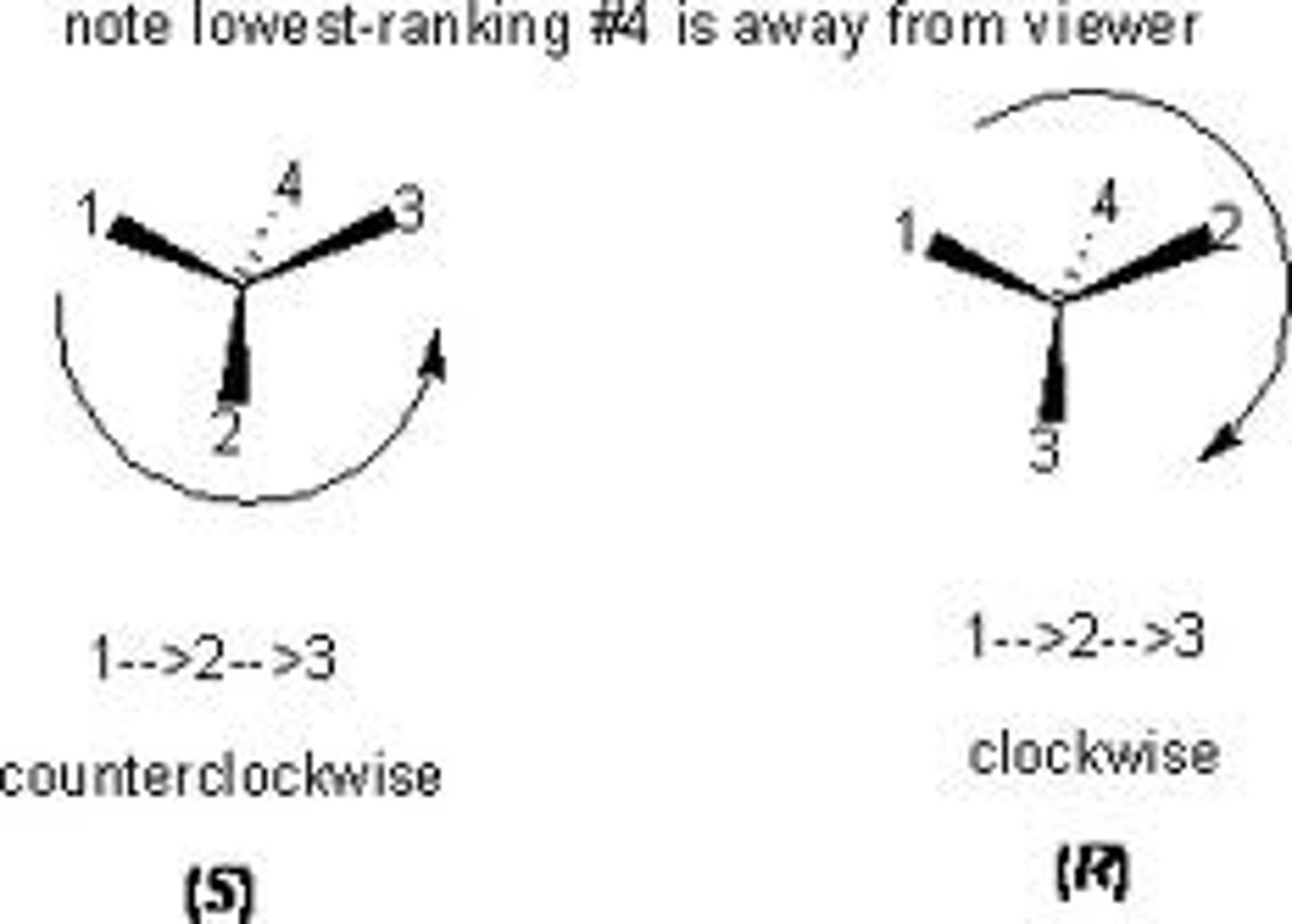
R
right, clockwise
S
left, counterclockwise
chiral compounds without chiral centers
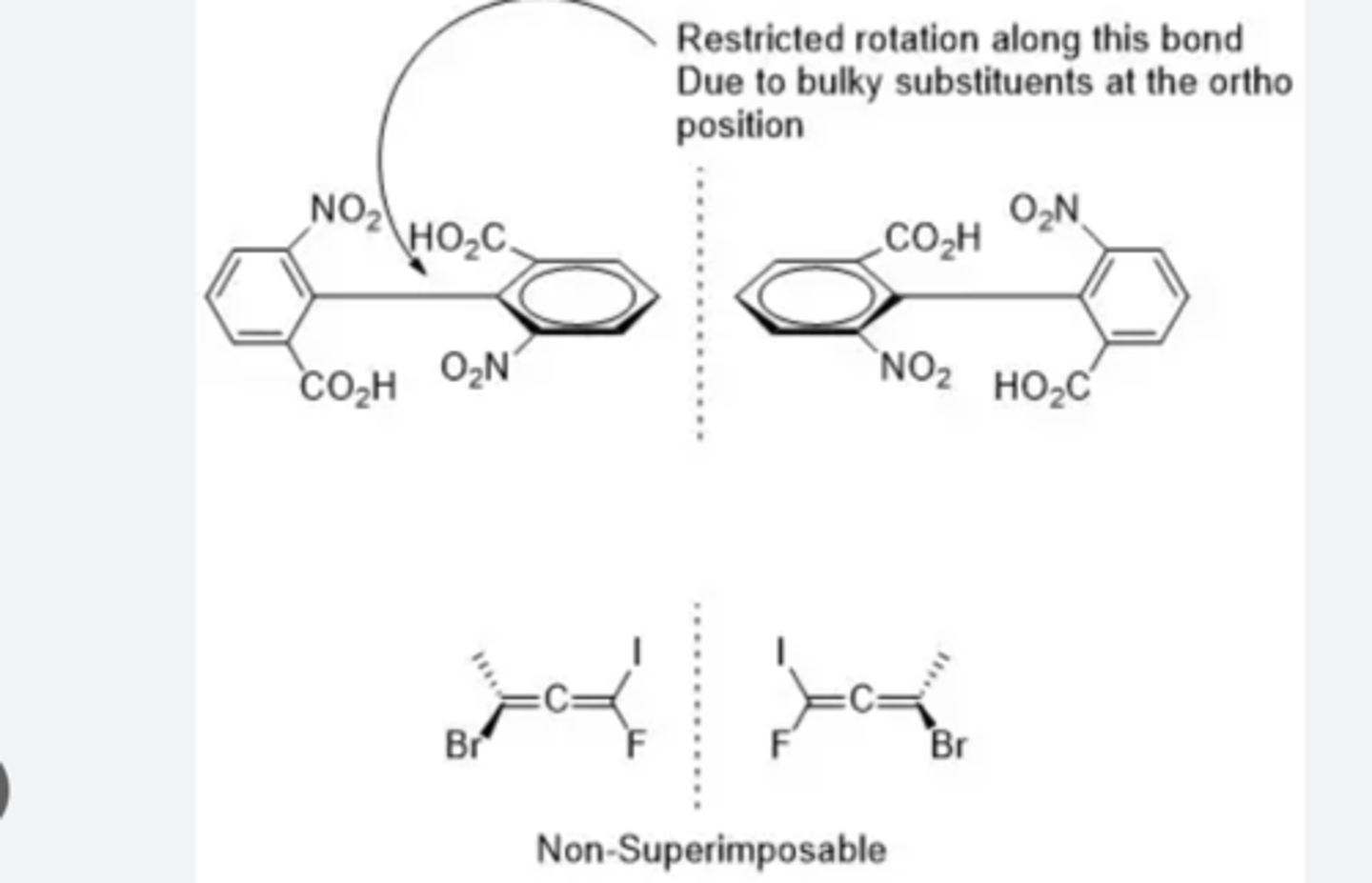
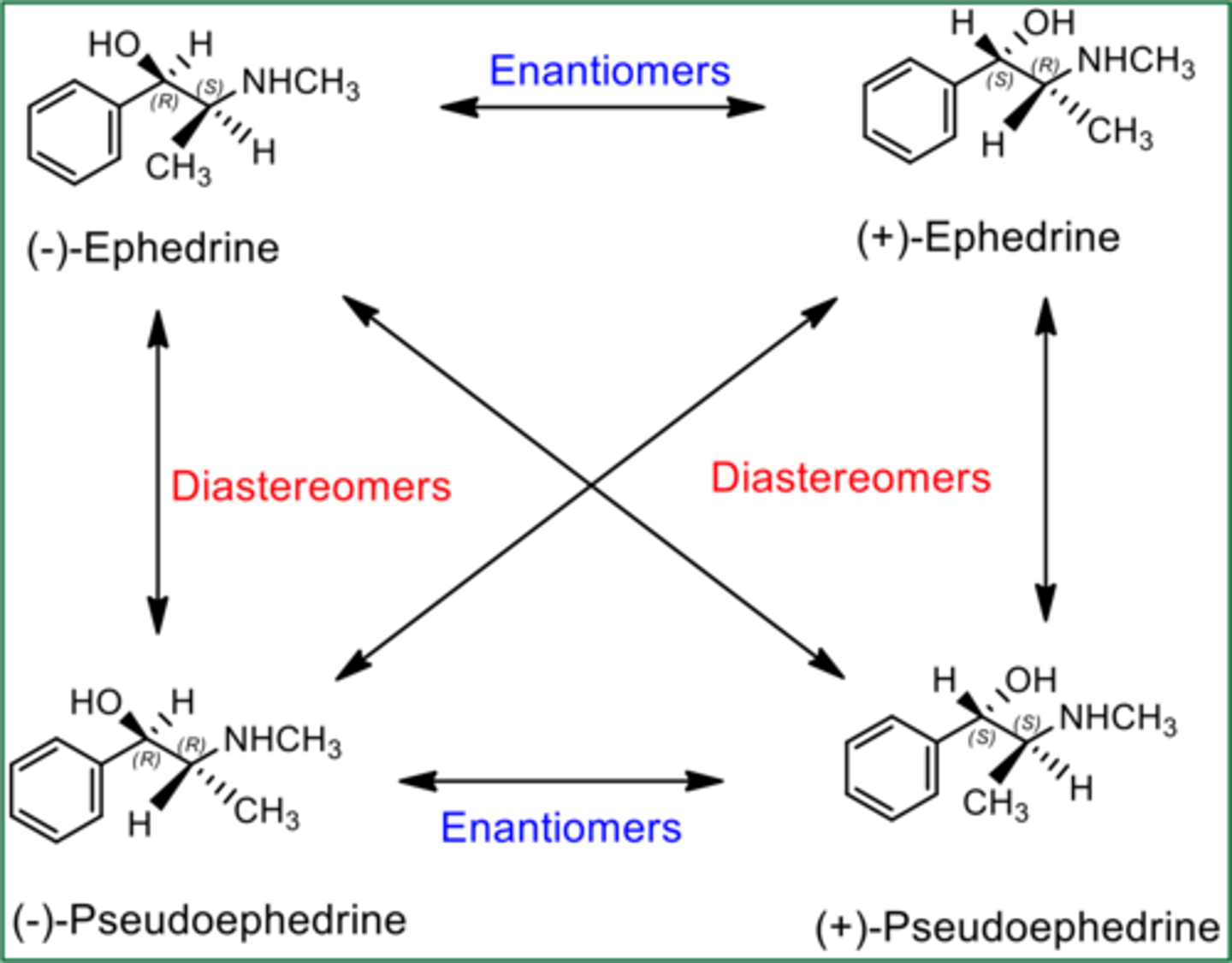
diastereomers
stereoisomers that are not enantiomers; maximum number of 2^n isomers
have different physical and chemical properties from one another and are separable
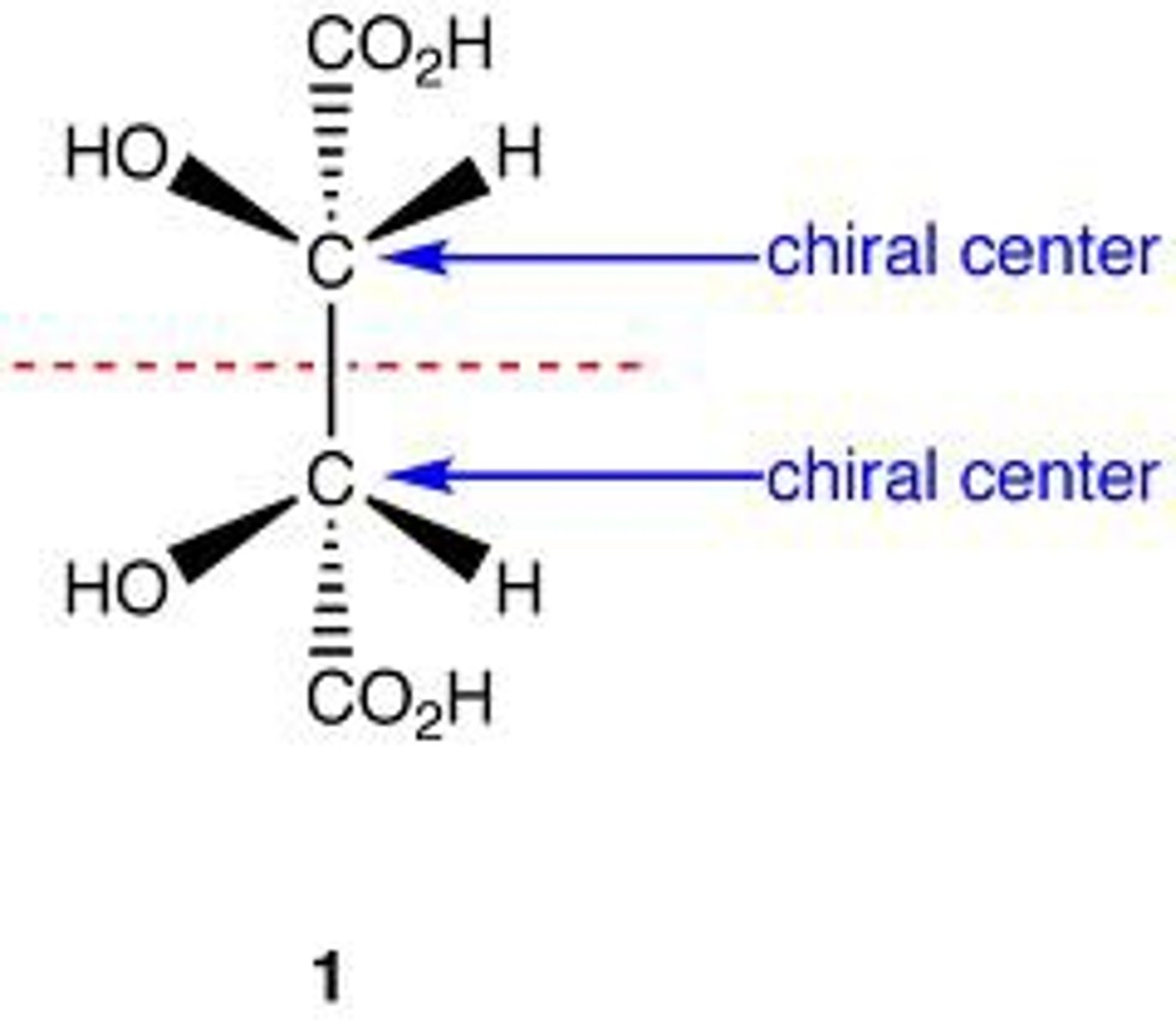
meso compounds
contain an internal plane of symmetry and are achiral (mirror image is identical to the original); maximum number of 2^n -1 isomers
types of chiral tests
optical rotation, odor, physiological behavior
unequal mixtures
aka not racemic
characterized by their enantiomeric excess
enantiomeric excess = (% maj enantiomer) - (% minor enantiomer)