Honors Physical Science Mid-Term
1/39
Earn XP
Description and Tags
semester 1,2025-debonis
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
How do you find Mass Number?
By adding the number of protons and neutrons in a nucleus.
whats an atomic number made up of?
number of protons in a nucleus.
what is an electron cloud?
Electrons are most likely to be found in the area outside of an atoms nucleus, called an electron cloud. Electrons are an energy levels in the electron cloud.
how many electrons are in the 1st energy level?
2 electrons
How many electrons are in the 2nd energy level?
8 electrons
How many electrons are in the 3rd energy level?
8 electrons.
Do protons and neutrons have the same mass?
yes, meaning one AMU is also equal to the mass of one nuetron.
Octet rule
atoms want 8 valence electrons
What is an isotope?
an atom of an element with the same number of protons but a different number of neutrons.
what is an ion?
an atom or molecule with a net charge, positive-cation, negative-anion.
what parts of an atom have mass?
protons and neutrons (not electrons)
What do atoms “want”
To have a full electron energy levels. Atoms with full electron energy levels are more stable and do not often react.

What is the atomic mass?
12.011
what is atomic mass?
The mass of an atom is so small that it needs its own unit. Atomic Mass Unit (amu) is the scale scientists use to measure the mass of atoms. One amu is equal to one proton.
The average mass of an elements atom, calculated by considering the different isotopes and their relative abundance.
How many electrons are in the 4th energy level?
18 electrons (e-)
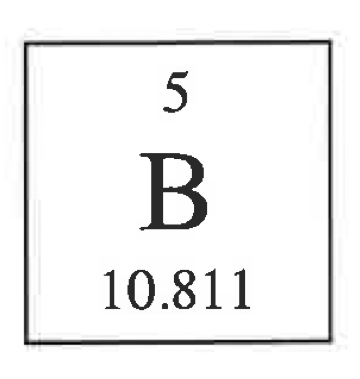
Whats the mass number if they’re 6 neutrons
11
In an electron-dot structure of an elements atom, the dots are used to represent__?
The valence electrons.
How do you calculate atomic mass?
You times the average mass(amu) by the % abundance.(you must move the dot 2 spots to the left) and then add all your answers together.
Example
Neon-20 19.9924 amu 90.84%
19.9924(.9084)=18.16109616
if a element has 8 protons but 9 neutrons its an__?
Isotope

What is the same in the photo above?
nuetrons
What is changed to form different isotopes?
nuetrons
what determines the size of the atom?
Electrons
What is changed to form different ions?
electrons
Has a mass of 1 amu
protons and nuetrons
What makes up the nucleus?
Protons and nuetrons.
1
mono
2
di
3
tri
4
tetra
5
penta
6
hexa
7
hepta
8
octa
9
nona
10
deca
Independent Vairables
The variable that is changed or controlled in a scientific experiment. It represents the cause or input.
Dependent Variable