Hematology 14: Anemias Pt 2 (Normocytic-Normochromic, Macrocytic)
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
function: Three Pathophysiological Mechanisms of anemia
production
ability to survive
maturation
Morphology: erythrocyte indices of anemia
MCV - cell size
MCHC - color (hemoglobin content)
Normocytic-Normochromic Anemia
Normocytic normochromic anemia is the type of anemia characterized by RBCs that are of normal size (normocytic) and have a normal hemoglobin content (normochromic)
•Most cases result from impaired RBC production.
Normocytic-Normochromic Anemia etiology
Most cases are consequences of other diseases such as •Chronic disease
•Hemolytic anemia
also depends on anemia type *hypo/hyperproliferative
Hypoproliferative Anemia
corrected reticulocyte count <2%
Hyperproliferative Anemia
corrected reticulocyte count >2%
normocytic-normochromic anemia pathophysiology
decreased rbc count, underlying disease or condition affects RBC production or lifespan
Pathogenesis Varies Depending on the Underlying Causes - 3 listed
Reduced Erythropoietin (EPO) levels:
Impaired kidney function affects the production of EPO
Increased proinflammatory cytokines:
Chronic inflammation (e.g., rheumatoid arthritis, lupus, inflammatory bowel disease) disrupts normal bone marrow function.
Bone marrow Infiltration/Invasion/RBC destruction:
Certain infections can invade the bone marrow or directly destroy RBCs
clinical presentation of normo anemia
same as anemia part 1 - fatigue, pallor and shortness of breath - need to look at underlying disease
reticulocyte count of normo anemia
May vary depending on the specific underlying condition
hemolytic anemias
Hemolytic anemia is a type of normocytic anemia characterized by an increased rate of red blood cell (RBC) destruction, which leads to low hemoglobin levels and increased bone marrow activity to regenerate RBCs.
Hemolysis: definition and lifespan of hemolytic cells
•Premature or increased rate of destruction of circulating RBCs
The lifespan of normal RBCs is approximately 120 days; in hemolytic anemias, it is reduced to 10-15 days
consequences of hemolysis
•Loss of RBCs
•Increased RBC production resulting in reticulocytosis
Damaged RBCs result in microspherocytes (hemolysis sign), elliptocytes, sickle cells, and red cell fragments (hemolysis sign)
Hemolytic anemias etiology
are broadly categorized into hereditary and acquired defects
•Hereditary or inherited: Examples include sickle cell anemia and thalassemia
•Acquired: Examples include autoimmune hemolytic anemia and infections
hemolytic anemia pathophysiology
•Increased RBC destruction, leading to a shorter lifespan
•Bone marrow compensates by increasing RBC production
clinical presentation of hemolysis
•Symptoms of anemia: shortness of breath, weakness, fatigue, etc.
•Jaundice, hematuria, and splenomegaly
•Fatigue and pallor
Hemoglobin Catabolism
•Once taken up by macrophages, RBCs are degraded, releasing hemoglobin.
•The globins are further broken down into amino acids and then used for protein synthesis.
•The Heme group (porphyrin + Fe) gets oxidized, producing biliverdin and releasing Fe.
•Biliverdin is reduced to unconjugated bilirubin.
•Unconjugated bilirubin is released into the plasma, where it binds albumin and is taken up by hepatocytes.
•Once released from heme, Fe has one of 3 fates.
Peripheral blood smear of Hemolytic Anemia
•Poikilocytosis
•Spherocytes
•Sickle cells
•Elliptocyte
bite cells
Hemolytic Anemia reticulocyte count
elavated
Hemolytic Anemia lactate dehydrogenase and indirect bilirubin
increased and increased
types of hemolytic anemia
G6PD Hemolytic Anemia and hereditary spherocytosis
G6PD Hemolytic Anemia
Caused by a deficiency in the enzyme Glucose 6 phosphate dehydrogenase (G6PD).
G6PD is a key enzyme in protection against oxygen radicals.
In its absence, RBCs are vulnerable to oxidative stress and damage, hb acumulation
Inclusion body: Heinz body
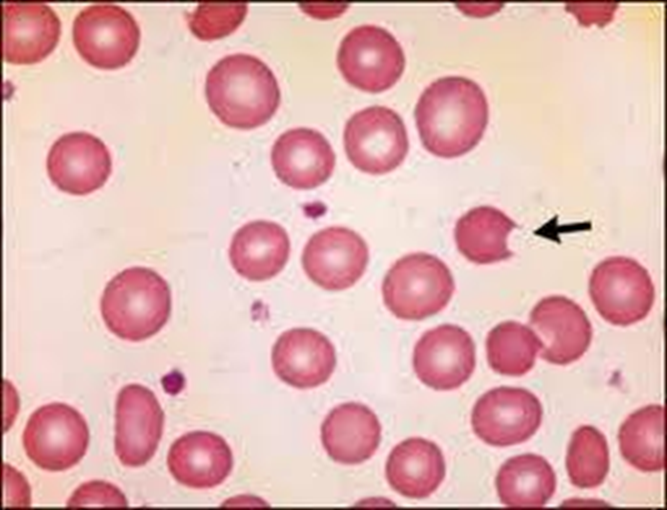
G6PD Hemolytic Anemia
cell bitten off, inclusion bodies (heinz) bitten off by spleen
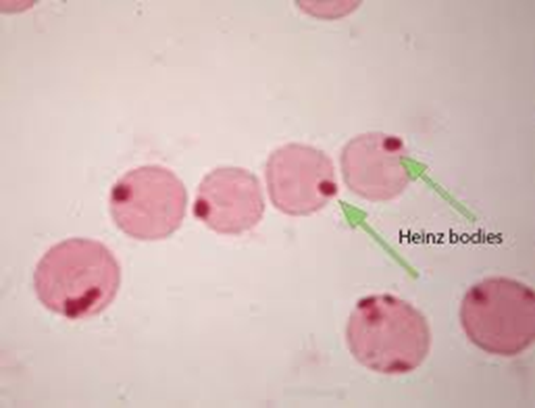
G6PD Hemolytic Anemia
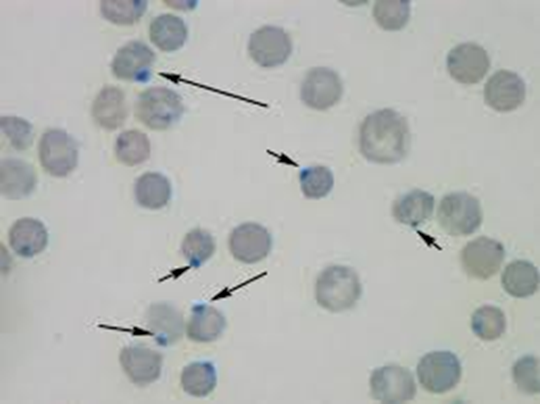
G6PD Hemolytic Anemia
sickle cell anemia
•Homozygous state caused by β-globin gene mutation. HbS
•This mutation causes the Hgb to become abnormally soluble and stable, resulting in SICKLING.
Why does sickling occur?
•HbS aggregates and polymerizes
Factors affecting sickling
Oxygen
HbA & HbF
Factors decreasing sickling
Oxygenation
HbA & HbF
Factors favoring sickling
•HbC & HbS
•Acidosis, fever, infections
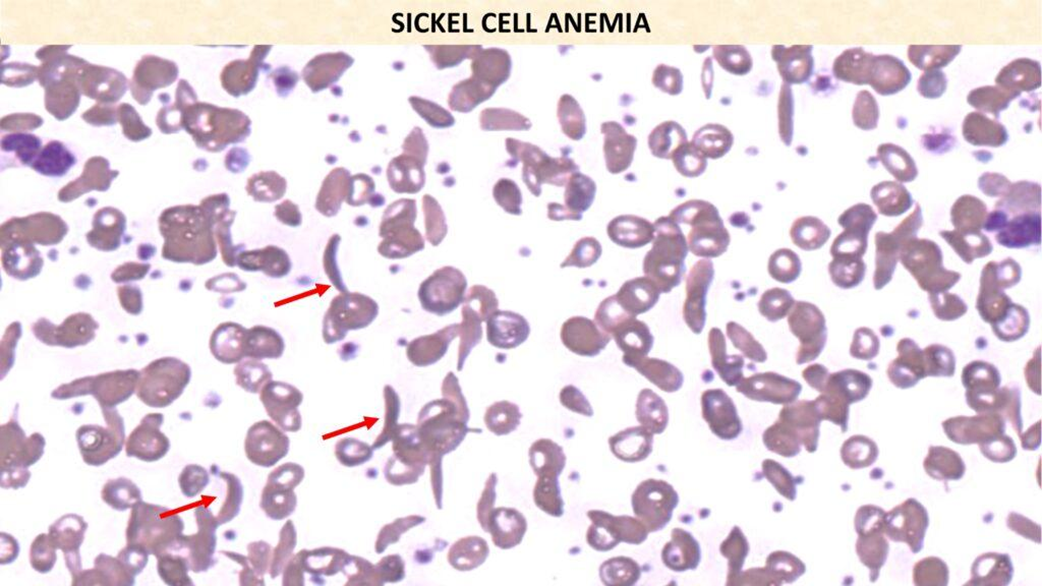
sickle cell anemia
clinical features of sickle cell anemia
Symptoms appear after 6 months as HbF is protective in the first 6 months of life.
•Chronic hemolysis – anemia
•Ischemic tissue damage resulting from occlusion of small blood vessels.
•Chronic hyperbilirubinemia- Jaundice
what is Sickle Cell “CRISIS
it is a sickle cell event where cells undergo sickling
what types is sickle cell crisis affected
Vasoocclusive/Pain crisis
aplastic crisis (bone marrow)
hemolytic crisis
Vasoocclusive/Pain crisis:
Episodes of hypoxic injury and infarction associated with severe pain in the affected region. The most commonly involved sites are the Bone, lungs, liver, brain, spleen, and penis.
what organs do sickle cell anemia affect (
Chronic Organ Damage
Spleen – Initially splenomegaly, Gradual loss of splenic function due to infarct- AUTOSPLENECTOMY (after overuse of spleen)
Bone - Osteomyelitis
Kidney – Renal infarcts/papillary necrosis, might need kidney transplant
Skin - Ulcers
Heart – Ischemia , cardiomegaly after long time of low o2 CCF due to long standing anemia