Phenols
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What are phenols
benzene rings with a OH group attached
What is the test for phenols
add phenol to iron (III) chloride and shake
solution goes from yellow to purple
Phenols are ________ acids and react with _______ _________
weak
strong alkalis
Phenols dissolve a little bit in water to form what
a phenoxide ion and a H+ ion - solution is weakly acidic
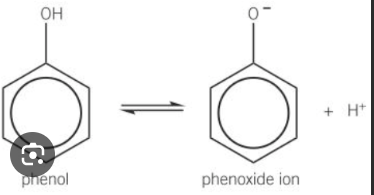
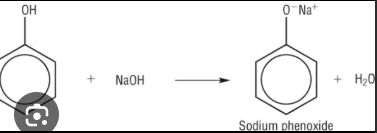
Phenols react with alkalis to form what
salt and water
Phenols do not react with _______ solutions
carbonate
Phenols react with acid anhydrides to form what
Ester and Carboxylic acid
Phenols do not react with ____ acids to form an ester
carboxylic
Describe how the OH group can act as an acid wait water to give the oxonium ioin
OH group acts as an acid - donates proton (H+) to water
forms H3O+ (Oxonium ion)
Recall the chemical test for carboxylic acid
react with sodium carbonate
produces carbon dioxide, water and a salt - fizzing from carbon dioxide