Environmental Engineering - Drink Water: Final Exam
1/66
Earn XP
Description and Tags
Final Exam concepts review
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
mass flux definition
rate of mass transfer
mass flux equation
m dot = J x A (flux density x area)
flux density definition
rate of mass transferred across a plane
flux density units
J = mass/time-area
3 mass transfer processes
advection, diffusion, dispersion
advection
“bulk flow” movement of a compound along with flowing air or water (J = C x v = concentration x velocity)
diffusion
net effect of random molecular movement caused by molecule’s thermal-kinetic energy. governed by Fick’s Law
diffusion equation
J = -D(dC/dx) = diffusion coefficient x concentration/position. Fick’s Law!
turbulent dispersion
mass transfer through mixing of turbulent eddies. dependent on Reynold’s number
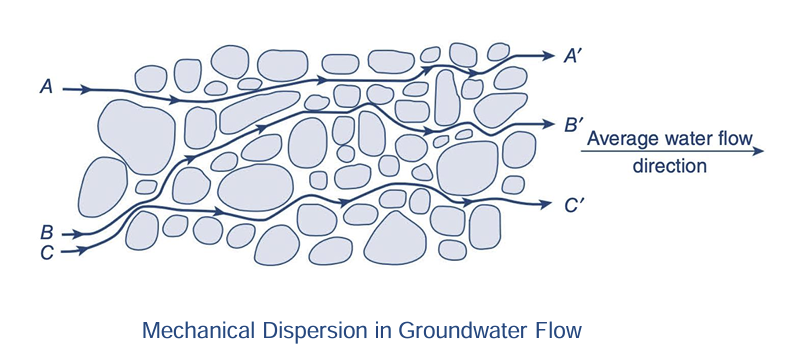
mechanical dispersion
variation in flow pathway and velocity of fluid particles with similar origin points
Darcy’s Law
rate of flow through a porous media
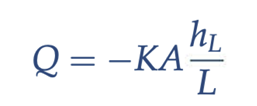
Darcy’s velocity
specific discharge
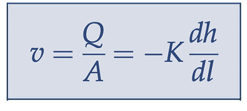
seepage velocity
seepage velocity
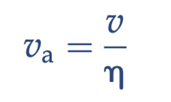
porosity (n) of an aquifer material

retardation coefficient
implies that a chemical that is undergoing sorption with the surrounding soil or aquifer material will travel at a slower rate than the average velocity (va) of the groundwater by a factor of Rf.
retardation coefficient equation
Kp - soil-water partition coefficient (L/kg)
n - porosity (unitless)
rohb - bulk density
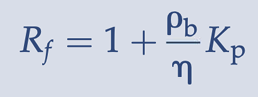
hardness in water
sum polyvalent metal cation (alkaline earth ions). given in mg/L as CaCO3
total hardness
TH = Ca2+ + Mg2+ = carbonate hardness + noncarbonate hardness
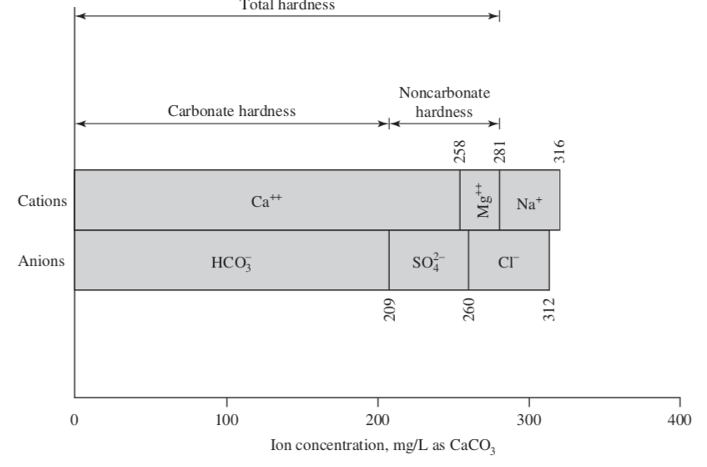
carbonate hardness (CH)
Ca2+ and Mg2+ associated with alkalinity anions (for example, HCO3- )
noncarbonate hardness (NCH)
Ca2+ and Mg2+ associated with nonalkalinity anions (for example, SO42- and Cl-).
lime-soda ash softening
A water treatment process that removes hardness from water by adding lime (Ca(OH)2 and CaO) and soda ash (Na2CO3) to precipitate calcium and magnesium.
reactions for lime-soda ash softening
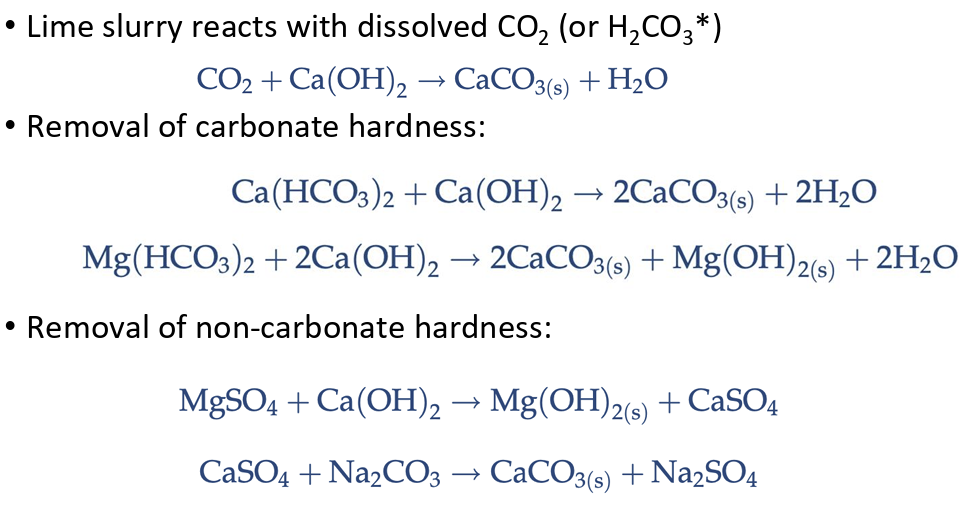
pH requirements for softening
removal of magnesium as Mg(OH)2 precipitate requires a pH value of at least 10.5. Therefore, extra lime (30–70 mg/L as CaCO3) in excess of the stoichiometric amount is added to raise the pH.
coagulation
water treatment process that involves adding chemical agents (coagulants) to destabilize suspended particles by enabling particles to come closer and stick together.
coagulant aid
substances that enhance the coagulation and flocculation process. insoluble particles like clay, diatomite, powdered activated carbon, fine sand.
flocculation
subsequent treatment step where gentle mixing encourages destabilized particles to aggregate into larger and heavier clusters (flocs), which can be more easily removed through settling or filtration.
particle stability
The tendency of suspended particles in a fluid (like water) to remain separated and resist aggregation or settling
repulsive electrical double layer (EDL) force
force between particles of the same charge. most particles in natural waters are negatively charged
van der Waals forces
attractive forces between particles
mechanism for C&F: Compression of the Electrical Double Layer (EDL)
Increasing ionic strength reduces repulsive forces by compressing the electrical double layer around negatively charged particles.
mechanism for C&F: Charge Neutralization
Negatively charged particles become destabilized and aggregate when their surface charges are neutralized by adsorbing positively charged ions or polymers.
mechanism for C&F: Adsorption and interparticle bridging
Nonionic polymers and long-chain low-surface-charge polymers form bridges between particles by adsorbing onto multiple particle surfaces, enhancing aggregation.
mechanism for C&F: Precipitation and Enmeshment
High doses of aluminum or iron salts form precipitates that physically trap particles, removing them through settling ("sweep floc")
types of coagulants
inorganic metallis coagulants
prehydrolyzed metal salts
organic polymers
natural plant-based materials
most commonly used coagulant
aluminum sulphate (Alum)

2 mechanism for particle aggregation
differential sedimentation and Brownian motion
granular (media) filtration
solid-liquid separation process for removal of colloidal and suspended particles
when is filtration used? what is the medium?
final particle removal process. sand or other media such as coal, activated carbon, or garnet.
slow sand filtration
A water treatment process using a bed of sand to remove suspended solids and pathogens through biological and physical processes.
requirements for slow sand filtration
low turbidity, regeneration (scraping of top sand layer)
rapid filtration
high-rate filtration using filtration and backwashing stages
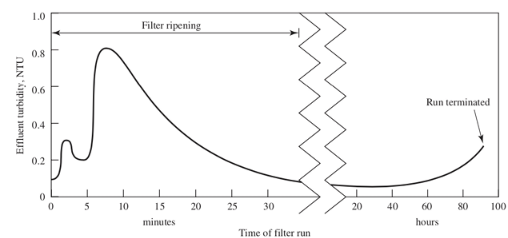
rapid filtration overview
sedimentation
the process of settling out suspended particles from water through gravity (majority of particles removed this way). works for particles with a density greater than 1000 kg/m³ (density of water)
2 types of settling
discrete and flocculant
discrete particle settling
particles are discrete and do not interfere with one another
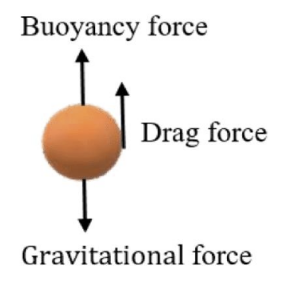
how to describe discrete settling velocity
Stokes’ law or Newton’s law
Reynolds number
ratio of inertial forces to viscous forces (low = laminar flow, high = turbulent flow)
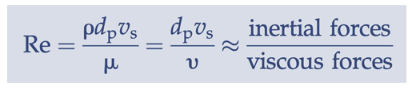
Stokes’ Law
applicable for spherical particles, Re < 1
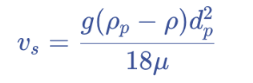
drag coefficient for Stokes’
a dimensionless number to quantify resistance of a particle moving through a fluid
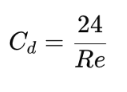
Newton’s Law
applicable for Re > 1 (transitional and turbulent flow)
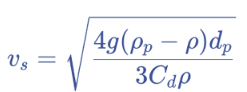
drag coefficient for Newtons
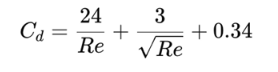
primary disinfection
inactivation of microorganisms in water
secondary disinfection
residual maintenance. maintaining a disinfectant residual in treated-water distribution system
common disinfectants
free chlorine (primary and secondary)
monochloramine (secondary)
chlorine dioxide (primary and secondary)
ozone (primary)
UV light (primary)
free chlorine
HOCl and OCL-
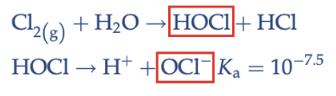
desired pH for free chlorine disinfection
pH = 7 because proportions of OCl decrease at lower pH and HOCl decreases at higher pH. HOCl is more effective so pH can be slightly lower than 7 if necessary
combined chlorines (chloramine)
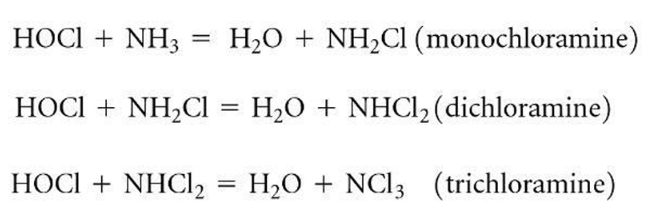
draw the chlorine dosage vs residual graph
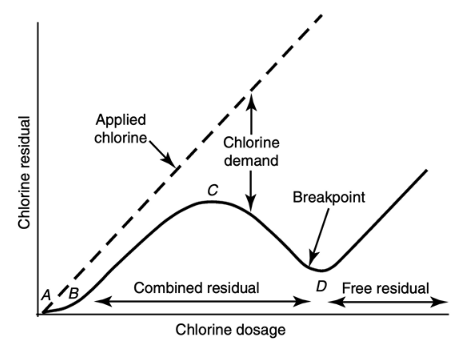
chlorination breakpoint
when combined chloramines are mostly oxidized. beyond the breakpoint, all added residual is free available chlorine
disinfection kinetics
N: Concentration of viable organisms
N0: Initial concentration of organisms
t: Disinfectant contact time
k: Disinfection rate constant
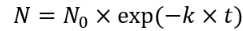
Chick’s law (derivative)
assumes the rate of disinfection reaction is pseudo first order with respect to the concentration of the pathogens being inactivated
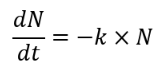
Chick’s law (using a natural log)
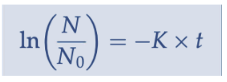
degrees of disinfection

disinfection dosage
(C x t product) inactivation of a microorganism is controlled by the concentration of the disinfectant and the time of contact
other disinfection factors
type of disinfectant
temperature
pH
viability of the microorganisms
turbidity
quantifying UV dose
I*t [mW/cm2 x s] (I is UV intensity)
chemical disinfectant most effective in inactivating microorganisms
C parvum