C2.1 Elements, compounds and mixtures
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
1
New cards
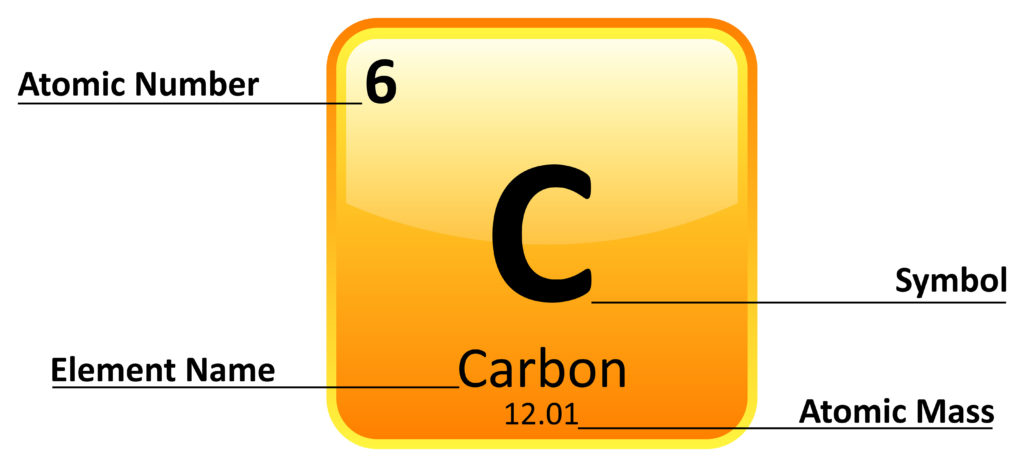
What is an element?
A substance made of atoms that all contain the same number of protons and cannot be split into anything simpler
Examples: hydrogen, carbon, oxygen
2
New cards
What is a compound?
A pure substance made up of two or more different elements chemically combined
It can only be split into different elements through chemical reactions

3
New cards
What is a mixture?
A combination of two or more substances (elements and/or compounds)that are not chemically combined
It does not require a chemical reaction to be split

4
New cards
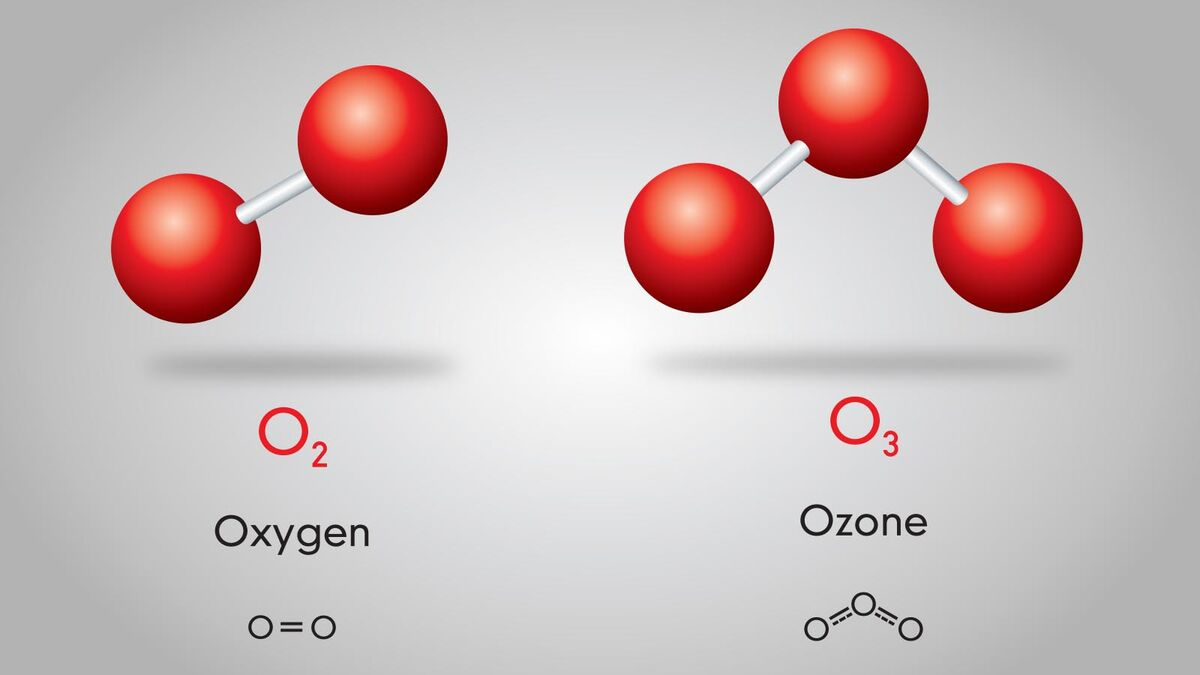
What is a molecule?
A group of two or more atoms (from the same or different elements) that are chemically bonded together