Chemistry - galvanic cells & fuel cells
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
68 Terms
galvanic cells
voltaic cell
a type of electrochemical cell
chemical energy is converted into electrical energy
spontaneous reaction
does not need to be driven by an external source of energy
salt bridge的图,自主交融,不需要外在因素相助
Inert electrode
当element本身不含有solid metal的时候,inert electrode将成electrode
e.g.
I2 (g)—> 2I- + 2e-
Sn (aq)
platinum: very expensive, prefer strong reductant and oxidant
graphite: no restriction (fragility, cant stand high voltage)
salt bridge
internal circuit
K+ —> Cathode
No3- —> Anode
Cation can transfer through salt bridge to cathode
anode produce cation flows to cathode
A salt bridge is a pathway that allows ion flow between half-cells, maintaining electrical neutrality and preventing charge buildup, which keeps the galvanic cell functioning.
electromotive force (emf)
potential difference exists between the two half-cells
Unit: Voltage (V)
measured by: Voltimeter
Conditions:
pressure: 100kpa
1M concentration
25 degree
systematic errors
cause readings to differ from the true value in a systematic manner so that when a particular value is measured repeatedly, the error is the same. Systematic errors result from limitations in the instrument itself or incorrect calibration, or inappropriate methods (include parallax)
repeated measurements
are made to reduce the effect of random errors (reduce the likelihood of mistakes)
mistakes
sometimes called personal errors. Mistakes should not be included in reporting and analysis as part of he ethical consideration of data handling. Rather, the experiment should be repeated correctly
uncertainty
the uncertainty of the result of a measurement reflects the lack of exact knowledge of the value of the quantity being measured.
random errors
affect the precision of a measurement and may be present in all measurements. Random errors are unpredictable variations in the measurement process and result in a spread of readings
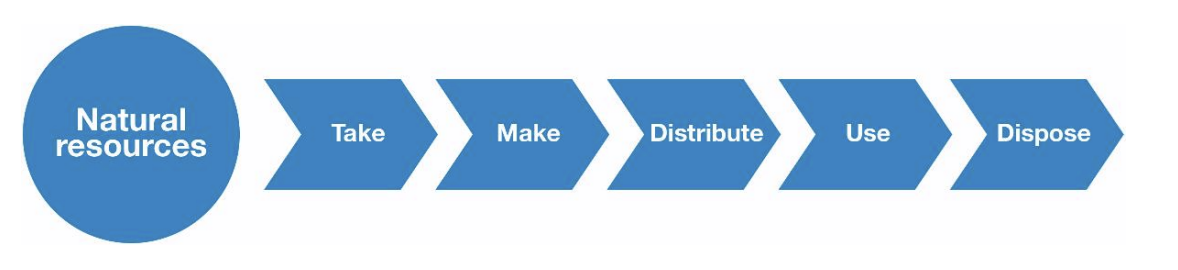
linear economy
Operates on a ‘take-make-dispose’ model, making use of resources to produce products that will be discarded after use. A simple representation is shown below.
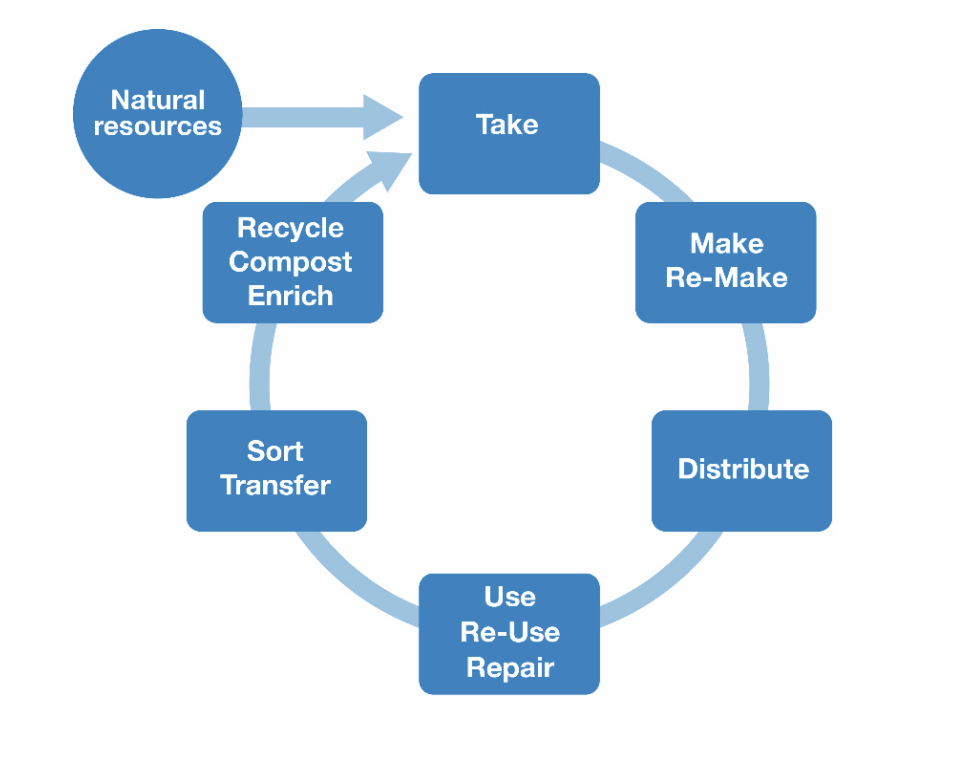
circular economy
A continuous cycle that focuses on the optimal use and re-use of resources from the extraction of raw materials through to production of new materials, followed by consumption and repurposing of unused and waste materials.
it is sometimes found that when an experiment is performed nothing happens, even though a reaction was predicted to occur. Suggest two reasons for the difference between prediction and what occured in the experiment
Activation Energy Barrier – The reaction may require more energy to get started than was available in the experiment, meaning the reactants did not have enough energy to overcome the activation energy.
Reaction Conditions – Factors like temperature, pressure, concentration, or the presence of a catalyst might not have been suitable for the reaction to proceed as predicted.
Fuel Cell (difference with primary / secondary cell)
Do not store energy, needs to supply from external sources
Continuous production of electrical energy from chemical energy
generally larger scale production of energy (buildings, vehicles)
can use gaseous reactants
continuous supply of reactants
continuous removal of products
electrode often acts as catalysts
porous electrodes
40-60% efficient
primary / secondary cell (difference with fuel cell)
contain stored chemical energy
portable sources of electricity
reactants are solid or aqueous (very rarely gases)
reactants inside of cell
products remain in cell
60-90% efficiency
porous diaphragm
acts as a salt bridge allowing passage of ions to keep solutions electrically neutral
Similarity - primary galvanic cell compared to a fuel cell
converts chemical energy directly to electrical energy
electrons flow from anode to cathode
difference - primary galvanic cell compared to a fuel cell
reactants will be used up in a primary cell while reactants are continuously being supplied in a fuel cell.
similarity - methane gas in a gas-powered power station compared to a methane fuel cell
overall equation of the reaction is the same
difference - methane gas in a gas-powered power station compared to a methane fuel cell
in a gas-powered power station, chemical energy is converted to thermal energy to mechanical energy before conversion to electrical energy while in the fuel cell, chemical energy is converted directly to electrical energy.
efficiency of fuel cells are much higher (~60%) than that of a gas-powered power station (~30-40%)
fuel cell - advantages
Direct conversion of chemical energy to
electrical energy —> high efficiency >60%
Hydrogen fuel cells produce water, heat and electricity as by-products. This means no carbon dioxide emissions.
Fuel cells can continuously supply electricity so long as fuel is supplied.
Fuel cells can use a variety of fuels.
Electricity can be generated in a stationary device or can be used to power mobile vehicles. Device does not need to be connected to the electricity grid.
Quiet (in comparison to a car engine)
fuel cell - disadvantages
Continuous supply of fuel required
Expensive to manufacture and electrolytes and catalysts can also be costly.
Generate direct current (DC) whilst many devices in homes and industries require alternating current (AC); an inverter is required.
Hydrogen must be very pure or catalysts can become poisoned, thus reducing their effectiveness.
Hydrogen is a dangerous substance to handle as it is combustible and explosive.
Hydrogen fuel sources are still in development:
Still sourced from fossil fuels
Electrolysis of water still inefficient, electricity comes from burning fossil fuels
But an area of intense research
Fuel supply chain not been developed – safe transportation of hydrogen and fuel stations.
Efficient and safe storage of hydrogen still in development.
what are fuel cells
A fuel cell is a type of galvanic cell that generates electricity from redox reactions cleanly, quietly and efficiently, with almost no polllution, from gaseous or liquid fuels such as hydrogen or hydrocarbons.
What accounts then for the loss of efficiency in fuel cells? Why not 100% efficient? Why still 60%
1. resistance in electrical wires. 2. loss of active materials / side reactions
overall equation for fuel cell
Fuel (g) + O2 (g) —> H2O (l,g) + CO2 (g) + heat
fuel —> anode
O2 —> cathode
H2 -> H2O (no CO2)
organic -> H2O + CO2
functions of electrode (fuel cell)
site of electron transfer
allow diffusion (reactants have to pass through and contact the electrolyte)
catalytic properties (decreases activation energy, increase rate -> efficiency
***reactive electrodes (take part in the reaction)
1. what are some factors that contribute to the energy efficiency of fuel cells
- direct conversion from chemical to electrical
- catalytic electrodes
- porous electrodes (higher surface area)
production of hydrogen
Hydrogen fuel cells do not contribute to emissions as water is the only chemical product. However, the product,on of hydrogen in the first place could contribute to emissions if non-renewable energy was used.
steam reforming
electrolysis of H2O (can be done without CO2 emission)
steam reforming
grey hydrogen —> worst for environment
95% of H is produced from fossil fuel
how does it work:
Steam reacts with fossil fuels (oil, natural gas, coal) at high temperature in the presence of nickel catalyst. The carbon monoxide produced can further produce hydrogen using a copper or iron
catalyst.
Limitations of steam reforming:
at high temperature
Waste heat from the product, on of hydrogen by steam reforming lowers its energy content.
Steam reforming also produces carbon dioxide.
However, the carbon dioxide produced at the site of steam reforming can be more easily contained, without releasing into the atmosphere.
green hydrogen
same reaction as steam reforming except using biofuels(可再生燃料)
Electrolysis of H2O (can be done without CO2 emissions)
blue hydrogen
2H2O (l) —> 2H2 (g) + O2 (g)
can be clean or dirt depending on energy source of electrolysis
-> if energy source is fossil fuel -> dirty
if energy source is renewable / no emissions -> clean
storage of hydrogen
liquify - carry more due to higher density
pressurise - dangerous - need to be larger to store the same amount of energy as liquid hydrogen tank
material base - adsorption of hydrogen onto the surface of metal hydrides as hydrogen molecules/atoms.
Green Chemistry Principles in designing of Products
1. Design for energy efficiency
a. Maximise energy efficiencies in the produc,on of electrical energy
2. The use of renewable feedstocks (covered in Redox Extension)
a. Microbial fuel cells
b. Redox Flow Bareries
humanitarian issues involving the mining of cobalt in Central Africa
excessive deforestation when mining
danergous condition for mines
use of child labour
pollution of wast supplies (environment —> human)
lower ethical standards
primary cell
disposable and designed not to be recharged
unrechargeable
single use
portable and convenient
secondary cell
rechargeable cells and designed to be reused many times.
electrolyte for the cell
alkaine - potassium hydroxide
acidic - phosphoric acid
methanol fuel cell (CH3OH) - polymer membrane
proton-exchange membrane fuel cell - solid polyperfluoro - sulfonic acid
solid oxide fuel cell - solid zirconium oxide
When the cell begins to produce electricity, the pH of the electrolyte near the cathode increases and eventually reaches a constant value. Explain why this occurs.
Why does PH increases?
At the cathode, a reduction reaction takes place, often involving the consumption of hydrogen ions or the production of hydroxide ions
This reaction produces OH⁻ ions, making the solution more basic, increasing the pH.
H⁺ ions are consumed, reducing their concentration and increasing pH near the cathode.
Why Does the pH Eventually Stabilize?
As the reaction continues, ion diffusion and mixing with the bulk electrolyte balances the pH.
The rate of OH⁻ production (or H⁺ consumption) reaches equilibrium with ionic transport, preventing further pH increase.
Thus, the pH rises initially due to the reaction but eventually stabilises as the system reaches dynamic equilibrium.
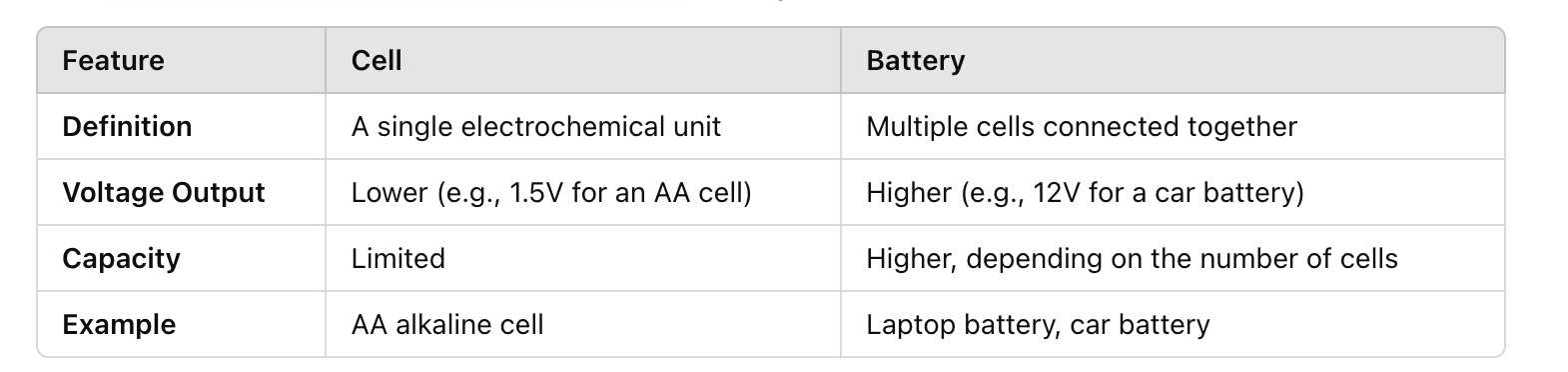
cell
A cell is a single electrochemical unit that converts chemical energy into electrical energy through a redox reacti
battery
A battery is a collection of two or more cells connected in series or parallel to provide a higher voltage or capacity.
difference between cell and battery
a battery consists of a number of cells connected together in series
benefits of porous electrode
increase surface area - larger reaction surface - increase efficiency
Allows better diffusion of reactants into the electrode, improving reaction kinetics.
improve ion flow - reducing resistance and improving conductivity.
maximising rate of reaction —> current / output of the cell
hydrogen fuel cells are better for the environment than petrol combustion engines
Hydrogen Fuel Cells vs. Petrol Combustion Engines: Environmental Benefits
✅ 1. Zero Harmful Emissions
Hydrogen fuel cells only produce water (H₂O) and heat as byproducts: 2H2+O2→2H2O+electricity2H_2 + O_2 \rightarrow 2H_2O + \text{electricity}
Petrol engines release CO₂, CO, NOₓ, and unburned hydrocarbons, contributing to climate change and air pollution.
✅ 2. Reduced Greenhouse Gas Emissions
Hydrogen fuel cells do not produce CO₂ during operation.
Petrol engines emit high levels of CO₂, a major contributor to global warming.
✅ 3. Higher Energy Efficiency
Hydrogen fuel cells have an efficiency of 50-60%, while petrol engines only achieve 20-30%, meaning less energy waste.
✅ 4. Renewable Potential
Hydrogen can be produced from renewable sources (e.g., electrolysis using solar or wind power).
Petrol relies on finite fossil fuels, which cause environmental damage through extraction and refining.
✅ 5. Reduced Air Pollution
Fuel cells do not produce smog-forming pollutants like NOₓ.
Petrol engines contribute to urban air pollution, harming public health.
Challenges of Hydrogen Fuel Cells
⚠ Hydrogen production can still generate emissions if sourced from fossil fuels.
⚠ Storage and distribution of hydrogen require infrastructure improvements.
Conclusion
Hydrogen fuel cells are cleaner, more efficient, and sustainable compared to petrol combustion engines, making them a better environmental choice when powered by renewable hydrogen. 🌱♻🚗💨
state two observations which could be made in the beakers to indicate that the cell is operating (Cu & Ag)
deposit of silver on the Ag electrode
dissolution of copper electrode
the blue colour of the copper(II) ion increases
the silver electrode increases in mass
the copper electrode decreases in mass
why does some electrolyte insuitable to use
because it might form percipitation
the DMFC is more efficient when run at high temperatures and pressures, and yet the conditions most commonly used are 50 degrees to 120 degrees and atmospheric pressure
suggest a reason to explain the use of these lower temperature and pressures
It is costly and requires more complex equipment to maintain high temperatures and pressures. Satisfactory performance can be achieved without the extreme, uneconomical conditions.
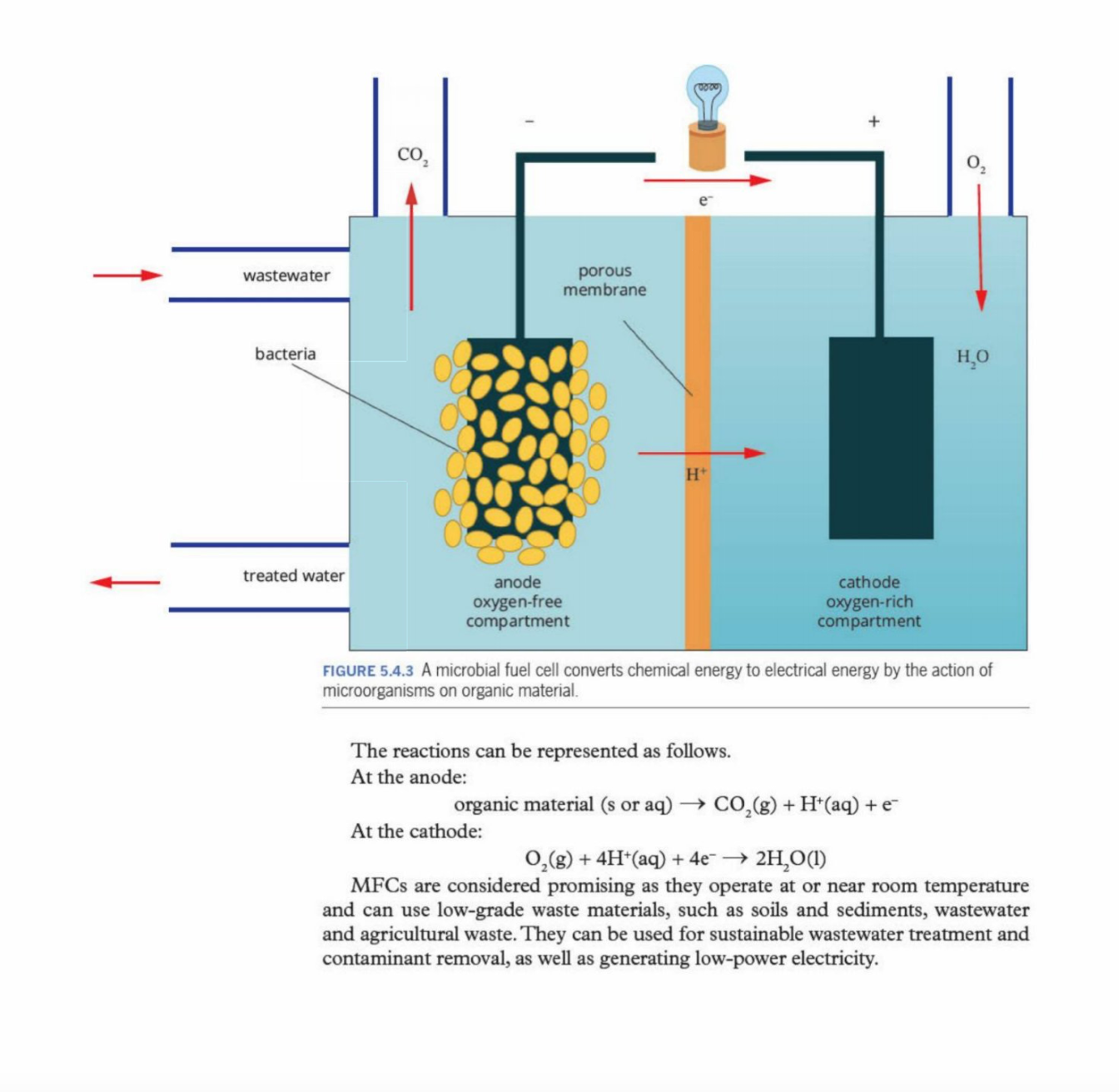
microbial fuel cell
a device that converts organic material to electrical
energy' by the action of microorganisms.
why does energy transfer from electrical to chemical energy during electrolysis of waater
Electrical energy is converted into chemical energy during electrolysis because energy is required to break water molecules and form high-energy products (H2\text{H}_2H2 and O2\text{O}_2O2). This stored energy can later be released in chemical reactions, such as in hydrogen fuel cells.
Why can batteries such as the molybdenum-air battery contain a much greater proportion. by mass. of reductant than conventional primary cells?
No need to store the oxidant (Oz) if it is supplied from air externally.
limits for electrical chemical theries
reaction has to be standard
aqueous solutions only
when current flow, it ease/difficult the reaction. e.g. H+ —> H2, hydrogen gas build up, difficult reaction
activity of solution
nature of the electrode surface
some reactions are so slow, that no observation
electrichemical theries
measure of tendency for a half cell reaction to push e- into the external circuit
secondary cell (application)
During charging, the reverse reaction occurs.
e.g. write half equation for the reaction occuring at the cathode during recharging
answer: inverse anode equation
During discharge, the NiOOH electrode acts as the cathode (positive terminal).
During recharge, the roles reverse, and NiOOH becomes the anode (still the positive terminal).
what is the polarity of r during recharge
positive
hydrogen economy
hydrogen could become a major source of energy and replace fossil fuels
purpose of proton exchange membrane in the fuel cell
To keep the reactants (CH3COOH and O2) separate in the cell
To prevent direct reaction between the reactants (CH3COOH and O2)
To allow the passage of H+ ions from the anode to the cathode
To prevent anions from passing through
requirment for electrode in fuel cell
conducting and porous
allow the hydrogen and oxygen to come into contact with the ions in the electrolyte and to allow the redox half reactions to occur at their surface.
biogas disad
Biogas has a similar limitation; the energy released per gram of biogas is less than that of natural gas because the methane content in biogas is significantly lower.
design for energy efficiency
In summary, the principle of "Design for Energy Efficiency" in fuel cells involves optimizing their design and operation to maximize energy conversion efficiency, minimize environmental impact, and utilize sustainable resources. This makes fuel cells a key technology in the transition to a more sustainable and energy-efficient future.
总而言之,燃料电池的“节能设计”原则涉及优化其设计和运行,以最大限度地提高能源转换效率,最大限度地减少对环境的影响,并利用可持续资源。这使得燃料电池成为向更可持续和更节能的未来过渡的关键技术。
use of renewable feedstock
In summary, the use of renewable feedstocks in fuel cells aligns with the principles of sustainability and green chemistry. It involves producing hydrogen and other fuels from renewable sources, integrating fuel cells with renewable energy systems, and developing sustainable materials and recycling processes. This approach not only enhances the environmental performance of fuel cells but also supports the transition to a more sustainable and resilient energy system.
Goal 7: Affordable and Clean Energy
Relevance: Fuel cells generate electricity through electrochemical reactions, often using hydrogen as a fuel, which produces only water and heat as by-products. This makes them a clean and efficient energy source.
Impact: By providing a reliable and sustainable energy source, fuel cells can help increase the share of renewable energy in the global energy mix, making energy more affordable and accessible while reducing environmental impact.
Goal 9: Industry, Innovation, and Infrastructure
Relevance: Fuel cells represent a significant technological innovation in the energy sector. They can be integrated into various industries and infrastructure projects to provide clean and efficient power.
Impact: The development and deployment of fuel cells can drive industrial innovation, support the development of resilient infrastructure, and promote sustainable industrialization.
Goal 11: Sustainable Cities and Communities
Relevance: Fuel cells can be used in urban settings for powering buildings, public transportation, and other community services with minimal environmental impact.
Impact: By reducing air pollution and greenhouse gas emissions, fuel cells contribute to creating healthier, more sustainable urban environments.
Goal 12: Responsible Consumption and Production
Relevance: Fuel cells promote efficient energy use and can be part of a circular economy, especially when hydrogen is produced from renewable sources.
Impact: They encourage responsible consumption and production patterns by reducing waste and optimizing resource use in energy production and consumption.
Goal 13: Climate Action
Relevance: Fuel cells produce low or zero emissions, depending on the source of hydrogen, making them a key technology in mitigating climate change.
Impact: By replacing fossil fuel-based energy systems, fuel cells can significantly reduce carbon dioxide and other greenhouse gas emissions, helping to combat global warming.
Goal 6: Clean Water and Sanitation
Relevance: While not directly related, the production of hydrogen for fuel cells through water electrolysis requires clean water. Efficient management of water resources is crucial for sustainable hydrogen production.
Impact: Ensuring clean water and sanitation supports the sustainable production of hydrogen, which is essential for the operation of fuel cells.
Goal 14: Life Below Water and Goal 15: Life on Land
Relevance: By reducing air and water pollution, fuel cells help protect ecosystems both on land and in water.
Impact: Lower emissions from fuel cells contribute to the preservation of biodiversity and the health of terrestrial and aquatic ecosystems.
Outline a safety issue with either the transport, storage or usage of hydrogen compared to methanol.

Durability
Operational reliability
Materials used
