The laws of thermodynamics
1/5
Earn XP
Description and Tags
0,1,2
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
What is the zeroth law of thermodynamics?
If two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
What physical property is defined by the zeroth law?
Temperature
What is the first law of thermodynamics?
The total energy of an isolated system is constant.
Energy is conserved but can transform between heat and work.
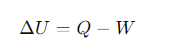
What physical property is defined by the first law?
Internal temperature
What is the second law of thermodynamics?
The total entropy of an isolated system can never decrease over time.
In any energy exchange, if no energy is added to or removed from the system, the system will tend toward a state of maximum entropy.

What physical property is defined by the second law?
Entropy