BIOL 2056 - cell adhesion
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
types of cell adhesion present in epithelial sheets
anchoring junctions
attach cell to other cells or cells to the ECM
tight/occluding junctions
seal cells together into sheets
gap/communicating junctions
allow exchange of chemical information
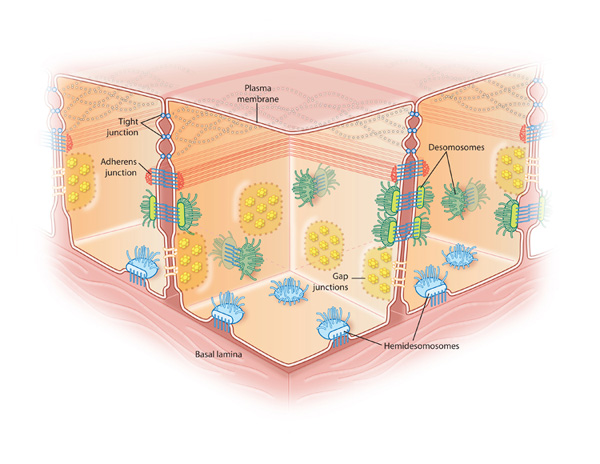
epithelial sheets porperties
anchored to teh basal lamina, therefore are polar
selectively permeable
delineate the organs and act as a barrier to leakage —> requires occluding junctions
anchoring junctions
can transmit mechanical info like stresses
types of anchoring junctions:
actin
cell-cell junctions called adherance junctions
cell-matrix junctions called actin linked cell matrix adhesion
intermediate filament attachment sites
cell-cell junctions called desmosomes
cell-matrix junctions called hemidesmosomes
occluding junctions
seal gaps between epithelia and prevent substance from moving from apical to basal side
the only way that things can pass through the barriers is through transporters
also prevents the diffusion of plasma membrane proteins, like transporters, which maintains the polarity of the cell
E.g the glucose transporter remains on the apical side of the cell, cant move to the basal side
structure of gap junctions
6 connexins make a connexon
homomeric means all the same connexins, whereas heteromeric connexins are diff connexins
heterotypic is different connexons, whereas homotypic is the same connexon
the different type of connexin determines the function and permeability
rapidly assemble and disassemble
signal relaying junctions - E.g chemical synapses
allow signals to be relayed across the plasma membrane at the site of cell cell contact
similar in principle to channel forming junctions
typically include anchorage proteins alongside proteins mediating signal transduction
families of cell adhesion proteins
CADHERINS
mediate cell-cell attachment
INTEGRINS
mediate cell-matrix attachments
Ig CAMS
immunoglobulin superfamily cell adhesion molecules
SELECTINS
bind carbohydrates
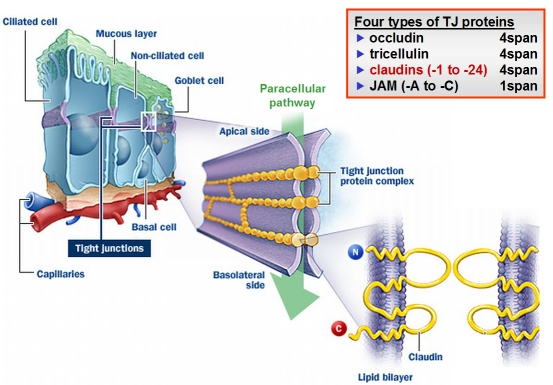
Tight junctions
types of protein present: all homophilic and all part of the CAM superfamily but have features which are specific to tight junctions. Dont individually have roles, only structural roles in maintaining tight junctions
CLAUDINS
4 pass transmembrane proteins that constitute the TJ strands
important for the strength of the TJs
JAMS
junctional adhesion molecules
single transmembrane proteins
OCCLUDIN
4 pass transmembrane protein localised at TJs
ZO
important for scaffolding and attaching claudins and occludins to the intracellular cytoskeleton
cadherins
proteins found in all multicellular animals
require Ca2+ to mediate cell adhesion
homophilic - cadherins will only bind to their type
lots of types can be found within tissues but are separated by spatial segregation
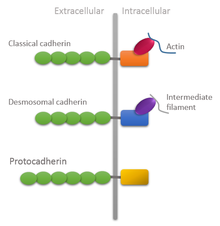
all cadherins have
extracellular domain, intracellular domain, form homodimers
the extracellular domain (the N terminus) sticks out and has multiple copies of the cadherin domain which bind to other extracellular N terminuses
C terminus is the intracellular domain
types of cadherin: different in different tissue types and have different structure too E.g: N cadherin in nerve cells, E cadherin in epithelial cells
function and bonding of cadherins
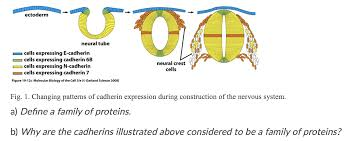
important for organsiation of tissues, such as the budding of the neural tube and the arrangement of tissue during embryogenesis
during neural tube formation, tissue on either side of the budding tube express the same cadherins whilst a section in the middle expressed different cadherins
H bonding between cadherins is relatively weak
the sheer no of cadherins make this interaction strong
multiple cadherin domains are held together by hinge regions
calcium binding to these hinge regions reduces the flexibility and causes them to reach out
homophilic interactions - cadherins on one cell type will only bind to the same type of cadherin on the other cell
activation of cadherins via wnt signalling
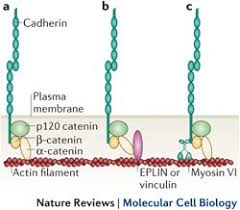
CADHERIN AND THE CYTOSKELETON
beta catenin forms a link between the intracellular cadherin domain and the actin cytoskeleton
the adherans junctions also have the additional related protein p120 catenin
BETA CATENIN AND WNT SIGNALLING
the ligand (wnt) binds to its receptor called frizzled, this activates dishevelled
active dishevelled prevents the degradation of beta catenin
beta catenin, when degraded by dishevelled, is important for replacing the transcriptional repressor groucho, which causes the expression of target genes
wnt signalling acts as a signalling pathway controlling 1000s of genes, but also occurs in the mediation of adherans juctions
breakdown of adherans juctions will lead to increased beta catenin —> increased transcription of genes
however, wnt signalling can also mediate the formation of adherans junctions
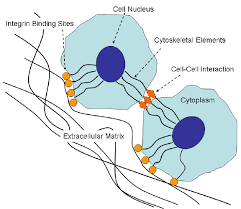
integrins structure and binding
STRUCTURE
have an alpha and beta subunit which bind to peptides in the intracellular domain
there’s a short intracellular C terminal and a large extracellular N terminal domain
the intracellular domain of the beta subunit is important for connecting to the cytoskeleton and mediating signalling
the extracellular domain will bind extracellular matrix proteins
BINDING
heterophilic
very strongly linked
needs to be dynamic —> integrins are broken and reformed when cells migrate
allosteric activation when integrin binds to ligands allows the switching between active and inactive states by conf change of IC and EC domains
activation of integrins
OUTSIDE IN ACTIVATION
binding of an extracellular ligand to an integrin results in binding to the cytoskeleton
RGD amino acid sequence is recognised by the integrin
this causes the alpha and beta subunits to mmove apart and frees up the tallin binding site which can bind tallin to the cytoskeleton
transmission of force via cytoskeleton
INSIDE OUT ACTIVATION
intracellular regulatory molecules such as phosphoinosotides activate tallin
tallin binds to the beta integrin chain
this causes teh extracellular domain of integrin to bind to extracellular ligands
PIP2 is then produced in response to extracellular signals
selectin
allows cells to roll across epithelium
the cell surface carbohydrate binding proteins are called lectins
single pass transmembrane protein
heterophilic interactions —> binds oligosaccharides
forms transient, weak interactions
control of when and where selectins and integrins are expressed regulates movement of WBCs
when the WBC reaches the site of injury, switches from selectin binding to integrin binding
types of selectins
L selectin - WBCs
P selectin - platelets and endothelial cells
E selectin - activated endothelial cells
Ig superfamily CAMs
mediate interactions between immune cells and endothelial lining
contain immunoglobular like extracellular regions
have a whole host of other functions depending on the type of CAM, such as barrier formation, cell signalling, synapse formation
EXAMPLE: NCAM
stands for neursal cell adhesion molecule
high levels of salicylic acid side chains which make them negatively charged which inhibits cell adhesion
can be made more/less sticky depensing on post translational modifications
NCAM has more subtle effects, more likely to be involves in the fine tuning of contacts
NCAMs can drive the growth of axon to the cell body, when the axon reaches the cell body, contact is mediated by cadherins which anchor it down
cell attachment in plants
pectin is important for cells to stick together
breakdown of pectin causes the ripening of fruit
Ca2+ presence and methylation state can affect how strong the pectin interaction is
theres not just one type of pectin, there are many diff types
pectin also has diff domains:
homogalacturonan —> is the backbone of the pectin
rhamnogalacturonan —> gives flexibility to the pectin
xylogalacturonan —> forms cross links