Exam 2: Glutamate and GABA
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
68 Terms
glutamate is a _________
amino acid (glutamic acid in ionized form)
what is glutamate’s main job?
excitatory NT of the brain
why is glutamate different from catecholamines?
With DA, Ach, or 5-HT you know the purpose of these NTs are always going to be used for neurotransmission when synthesized; GLU is different because it also has roles in protein synthesis and cellular metabolism (not always used as NT)
T or F: All neurons and glial cells have lots of GLU, but those that use it as an NT have even higher concentrations of GLU
true: all neurons have GLU but concentration differs
T or F: GLUergic neurons store all GLU in same place no matter its purpose
F: Gluergic neurons are thought to segregate the pool of GLU that they use for transmission from the pool of GLU used for other cellular functions.
how is GLU synthesized?
can come from many diff chemical reactions but most is derived from breakdown of glucose
glutamine is often the precursor for glutamate when glutamate is going to be used as an NT
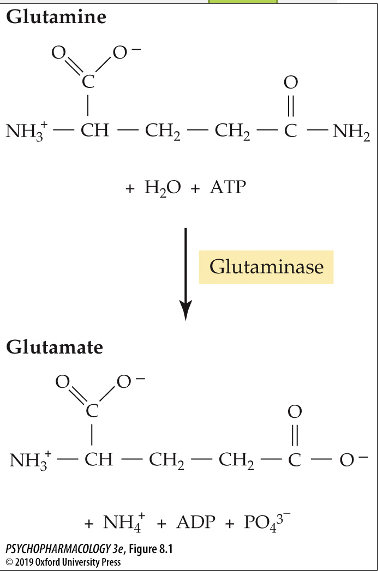
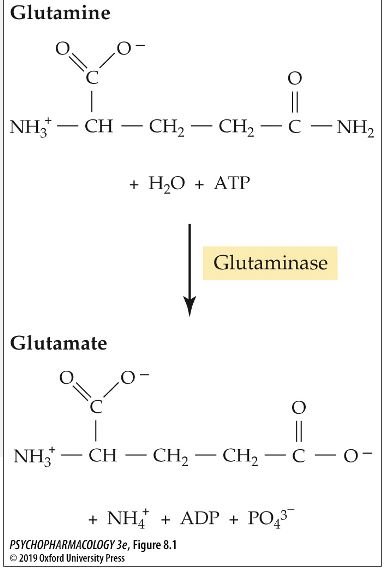
what enxyme converts glutamine to glutamate?
glutaminase
how is glutamate stored?
in synaptic vesicles via packaging by vesicular glutamate transporters (VGLUT)
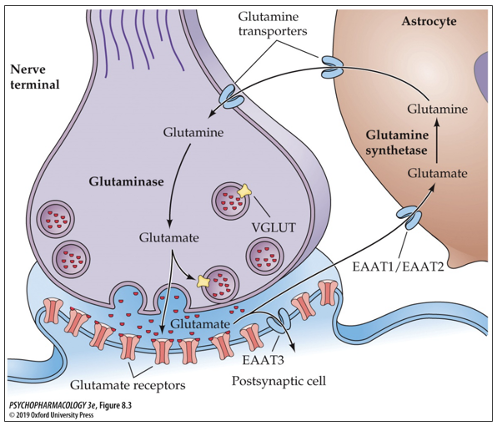
what are the 3 types of glutamate transporters? where are they located?
VGLUT1 located in cortex and hippocampus
VGLUT2 located in subcortical structures
VGLUT3 located in inner hair cells of cochlea
what role do astrocytes play in GLU transmission?
astrocytes release GLU
take up GLU from extracellular space
express GLU receptors
respond to activation of GLU receptors
how do astrocytes release GLU?
first they are stimulated by intracellular rise of Ca2+
some astrocytes express VGLUT on small vesicle-like structures
some release GLU through channels in the membrane
how is GLU inactivated?
mainly via reuptake into the nerve terminal via excitatory amino acid transporters (EAAT 1-5)
Prolonged high levels of GLU in the extracellular space is _______, producing excessive neuronal excitation and cell death
dangerous
transporter name for GLU reuptake
EAAT1-EAAT5
which EAAT transporter is most abundant?
EAAT2; accounts for 90% of total GLU uptake in the brain
how important is EAAT2?
very important; when knocked out in mice, these mice were underweight and died at much earlier age (died from seizure activity and excitotoxicity)
where is glutamate metabolized? where does it go from there?
metabolized in astrocytes then transported out of astrocyte into neuron via glutamine transporters
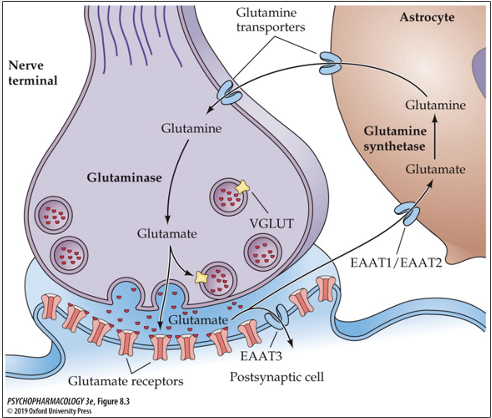
what enzyme metabolizes glutamate? what does it do?
glutamine synthetase; converts glutamate to glutamine
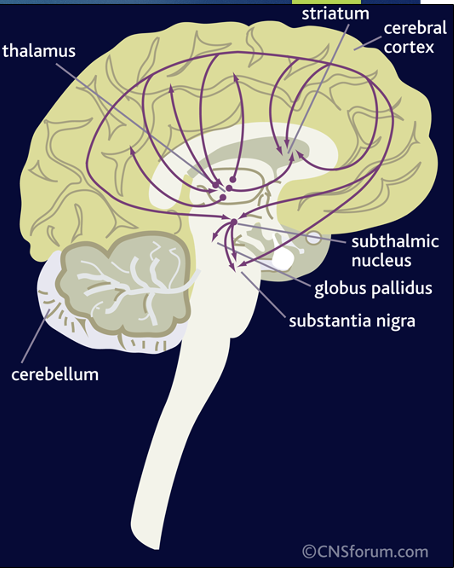
GLU brain pathways/projections
pyramidal neurons have cell bodies in the midbrain and subcortical structures that project to the striatum, thalamus, limbic structures, and brainstem regions
what type of receptor are the GLU receptors? how many subunits do they have?
ionotropic; 4 subunits
what are the three subtypes of GLU receptors? how are they named?
AMPA
Kainate
NMDA
*named based on the agonist that binds to them
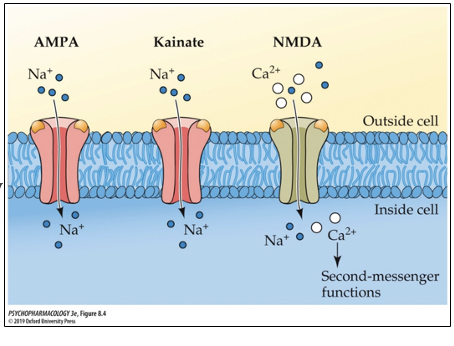
GLU receptor subtypes: AMPA
agonist is AMPA; mediates most fast, excitatory responses to GLU; has low affinity for GLU (many receptors are still AMPA despite this)
GLU receptor subtypes: Kainate
agonist is kainic acid
higher affinity for GLU than AMPA but slightly lower affinity than NMDA
GLU receptor subtypes: NMDA
agonist is NMDA
highest affinity for GLU
what makes NMDA receptor different from AMPA and Kainate receptors?
permeable to both Ca2+ and Na while the other two are only permeable to Na
Two different NT’s are required to activate the NMDA receptor: Glu and Glycine/D-serine
Has two additional binding sites inside the receptor channel (one binds Mg2+ and one binds several other non-competetive antagonists (PCP, ketamine, MK-801)
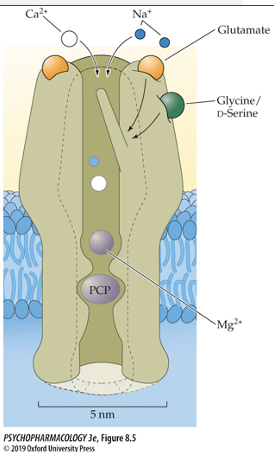
process of NMDA receptor activation
cell membrane starts at resting potential (-70 mV) and Mg2+ is blocking the channel
Cell must be depolarized via activation of another receptor like AMPA (this allows Mg2+ ions to detach from receptor and unblock channel)
Channel will only open. if GLU and co-agonist (Glycine) are also bound
what cognitive diseases have been associated with dysregulation of GLU receptors?
intellectual disability
ASD
Schizophrenia
proposed treatment for neuropsychiatric disorders resulting from dysregulation of GLU receptors?
To treat, you might use positive allosteric modulator that binds to AMPA, cholinergic, or nicotinic receptors; allows depolarization of membrane to dislodge Mg2+ and activate NMDA receptor
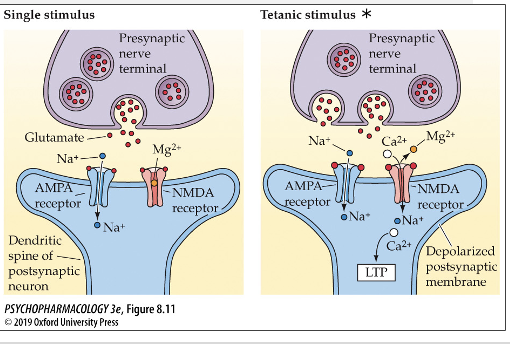
GLU and strengthening of synapses
if a presynaptic cell is consistently releasing GLU and creating strong activation of NMDA receptors in postsynaptic cell receiving GLU, this can lead to strengthening of that synapse
Long term potentiation (LTP) and GLU
A persistent (at least 1 hour) increase in synaptic strength produced by a burst of activity in the presynaptic neuron.
A postsynaptic cell will respond more easily to a presynaptic cell if the presynaptic cell is continuously releasing neurotransmitters onto that particular postsynaptic cell.
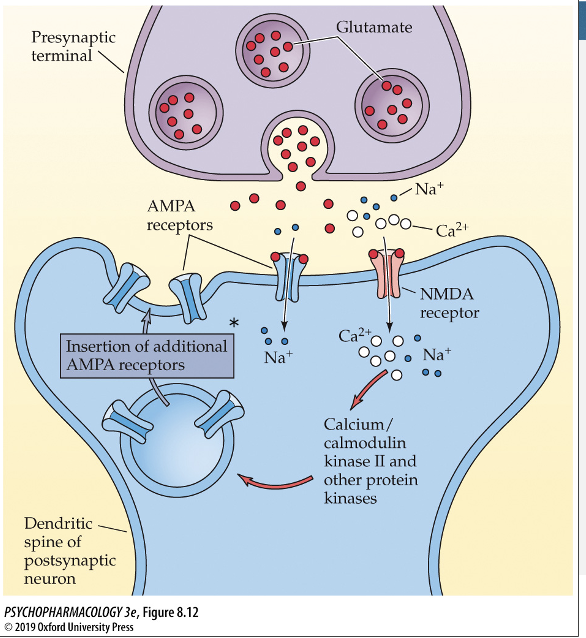
Resulting in larger EPSPs to the same amount of
neurotransmitter releaserate of AMPA receptors inserted into the membrane also increases and enhances the sensitivity to GLU on postsynaptic cell
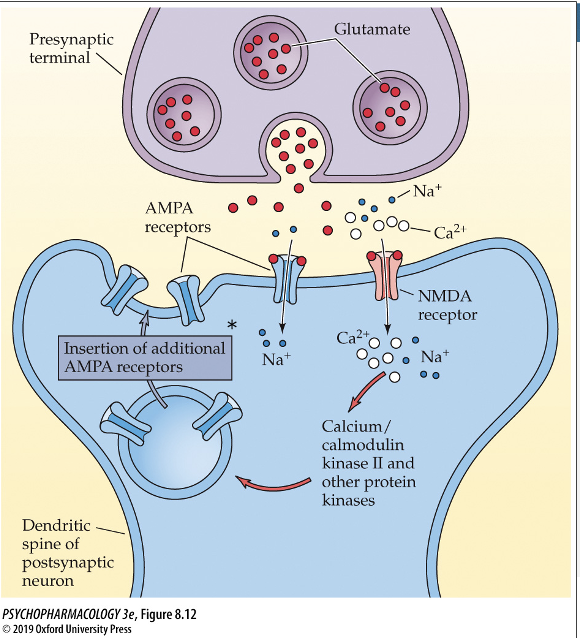
Long term depression and GLU
postsynaptic cell becomes less sensitive to GLU because GLU is not being released from the presynaptic cell very often; also a withdraw of AMPA receptors from the membrane which decreases sensitivity to GLU
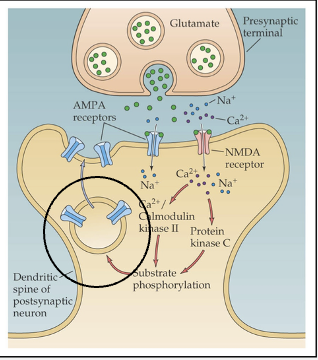
forms of LTP
early LTP: lasts only a few hours
late LTP: lasts days to months
where are AMPA receptors located when they aren’t in the membrane?
made in the cell body (ER)
sent to perisynaptic sites (where they hang out when not in membrane)
increase in Ca2+ dislodges them from perisynaptic site durting LTP and they move to the membrane; can return to perisynaptic site when signaling decreases
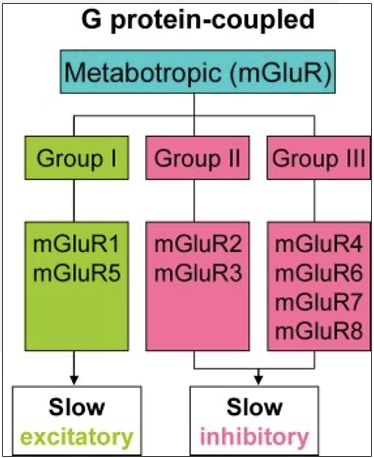
types of metabotropic GLU receptors
Group 1: Mostly postsynaptic, excitatory
Group 2 and 3: Presynaptic (autoreceptors); inhibitory
how do group 1 metabotropic receptors for GLU work?
activate phosphoinositide (PPI) to stimulate phospholipase C (PLC), forms inositol triphosphate (IP3) and diacylglycerol (DAG) as 2nd messenger
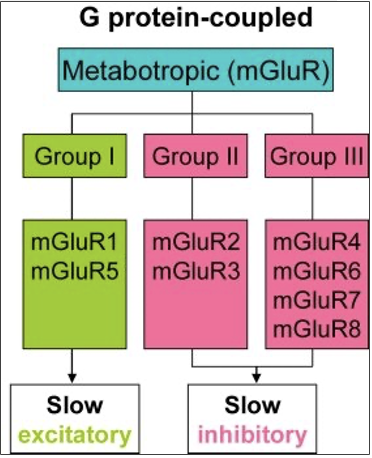
how do group 2 and 3 metabotropic receptors for GLU work?
inhibit adenlyl cyclase to inhibit cAMP
excitotoxicity hypothesis
postsynaptic receptors (NMDA, AMPA, Kainate) are overly stimulated by excessive exposure to GLU (and other excitatory amino acids) and have no ability to reset; causes negative effects and death of these neurons
necrosis vs. apoptosis
necrosis: not planned cell death/lysis; cell does not have enough energy for apoptosis; when cells lyse, surrounding neurons are negatively impacted by the calcium and GLU that spill out of lysed cell creating a positive feedback loop
apoptosis: programmed cell death
domoic acid poisoning
domoic acid is a toxin made by several species of marine algae that is taken up by shellfish, crabs, and fish
toxin is excitatory and binds to glutamatergic receptors
when humans/wildlife eat this seafood, they also ingest the toxin and could die
symptoms of poisoning: headache, dizziness, muscle weakness, mental confusion, permanent loss of short-term memory
amyotrophic lateral sclerosis (ALS)
A fatal neurological disorder involving a slow but progressive degeneration of motor neurons in the spinal cord and cortex (this is a chronic, slower-acting excitotoxic process)
patients have shown abnormalities of GLU system
Riluzole: only treatment available, is believed to exert its therapeutic action by reducing GLU release.
is GABA an amino acid?
yes, it is a member (with glycine) in a small family of inhibitory amino acid NTs; one of principle inhibitory NTs in the brain
what type of molecule does GABA serve as?
NT and co-transmitter
GABA synthesis
synthesized from glutamate (GLU) by glutamic acid decarboxylase (GAD)
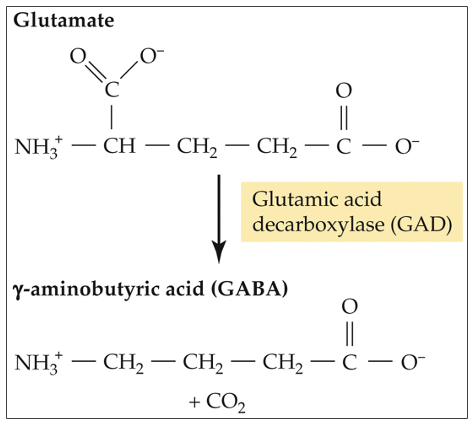
what results from a significant reduction in GABA synthesis?
convulsions (seizures)
how is GABA stored?
in synaptic vesicles via GABA transporter VGAT
T or F: Glycine and GABA use separate transporters for vesicle storage
F: VGAT is the same transporter for GABA and Glycine so sometimes it is also called VIATT (vesicular, inhibitory amino acid transporter)
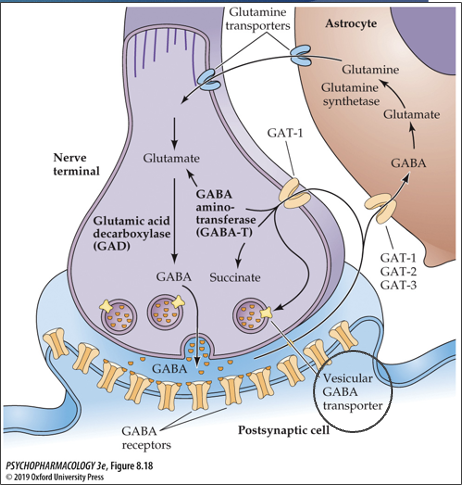
how is GABA inactivated?
reuptake into nerve terminal via GAT-1, GAT-2, and GAT-3 transporters
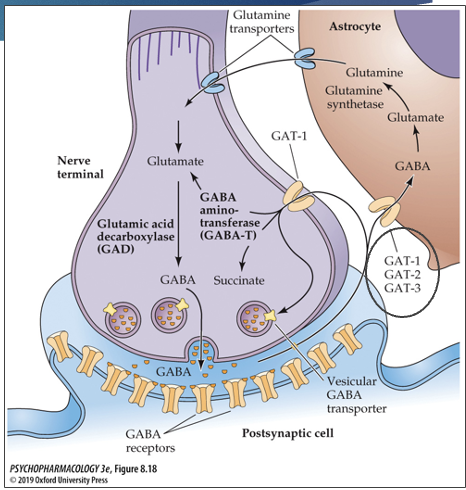
where are GABA reuptake transmitters located?
GAT-1: neurons and astrocytes
GAT-2: neurons and astrocytes
GAT-3: astrocytes
where is GABA metabolized?
both nerve terminal and astrocytes (uses different enzymes for break down based on location)
what enzyme is used to break down GABA in the nerve terminal? what does it break GABA down into?
GABA aminotransferase (GABA-T); breaks GABA down to glutamate/succinate (GLU)
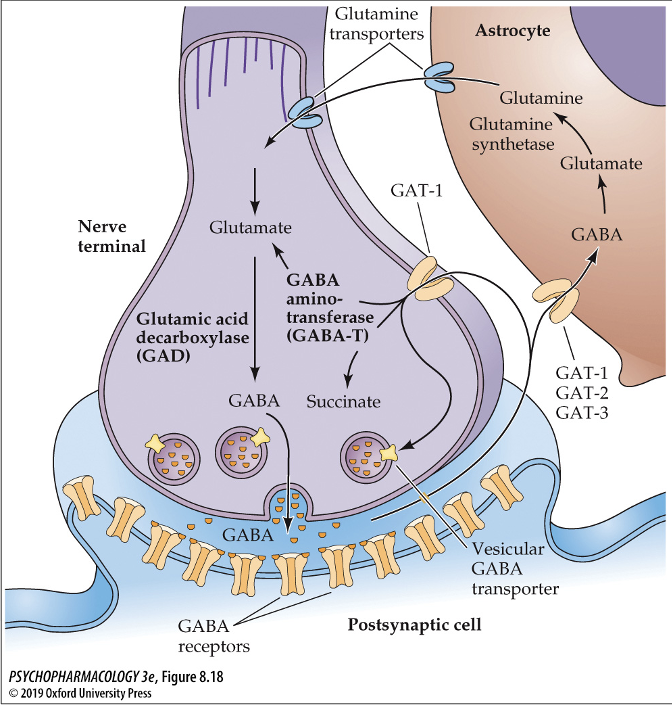
what enzyme is used to break down GABA in the astrocytes? what does it break GABA down into?
GABA aminotransferase (GABA-T); GABA → glutamate → glutamine synthetase → glutamine → glutamine is then transported out of astrocytes and into neurons via glutamine transporters

which drugs have been used as treatments for epilepsy? what is their method of action?
Tiagabine (Gabitril) and Vigabatrin (Sabril); both lead to build-up of GABA to decrease seizures
GABA pathway
GABA is pretty much everywhere in the brain like GLU
nerve terminals in hippocampus, cerebral cortex, and substantia nigra use GABA as NT (both projection and interneurons)
Cerebellum, striatum, globus pallidus, and olfactory bulbs are rich in GABA
GABA A receptor
ionotropic receptor with 5 subunits
activation (GABA binding) causes an inclux of Cl- ions which hyperpolarizes the postsynaptic cell
acts as a check on NS: if a cell was previously depolarized by an excitatory stimulus, more Cl- ions will flow through the channel to blunt depolarization and prevent cell from firing again too soon
GABA A receptor and epilepsy
changes/reversal in GABA A receptor from inhibitory to excitatory (Cl- flowing out instead of in) can cause epilepsy
what drugs are GABA A receptors sensitive to?
some CNS depressant drugs: anxiolytic (anti-anxiety), sedative-hypnotic (seducing and sleep-inducing), anticonvulsant, benzodiazepines and barbiturates
principal mechanism of action for benzodiazepines and barbiturates
positive allosteric modulation of GABA A; they bind to other sites on the GABA A receptor and increase anti-excitatory effect
barbiturates are a GABA A receptor ________ (agonsit/antagonist)
agonist; work by increasing the length of time the Cl- channels remain open
problem with barbiturates
they increase their effect when combined with other depressants like ethanol; when two positive allosteric modulators bind to GABA receptor this can result in overdose
barbiturates can activate the GABA A receptor on their own (without GABA) and usually GABA is still required for other positive allosteric modulators to become active
benzodiazepines are a GABA A receptor ________ (agonsit/antagonist)
agonist; works by increasing the frequency at which the Cl- channel opens
why are benzos safer than barbiturates?
while they do act potentiatively with other depressants like barbiturates, benzos cannot open the channel on their own in the absence of GABA
T or F: you cannot overdose on benzos
False; you can but it takes much more drug than barbiturates
benzo drug names typically end in ___ and ___
lam and pam
what can happen with chronic administration of GABA A positive allosteric modulators (like benzos)?
can lead to development of tolerance, dependence, and withdrawal symptoms if drug treatment is suddenly terminated
what type of receptor is the GABA B receptor?
metabotropic receptor
where are GABA B receptors located?
both presynaptically and postsynaptically
presynaptically: autoreceptors that reduce NT release through inhibition of Ca2+ channel opening
postsynaptically: inhibits neuronal firing through stimulation of K+ channel opening
why is GABA B different from other metabotropic receptors?
they are heterodimers; requires two different subunits to work properly (B1 and B2 come together to form the full receptor)
how does GABA B subunit work?
B1 subunit is under the cell surface attached to the ER
B2 subunit brings the B1 subunit to the cell surface by masking B1’s ER retention sequence via coil-coiled domain
B1 and B2 subunits join together; B1 has the site for ligand to bind and B2 is attached to the G protein and has sites for allosteric modulators to bind