Chemistry 101 - chapter 1 - Atoms and isotopes
1/39
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
40 Terms
What are the 3 states of matter and what about their shape and vlome
Solids: relatively rigid and have fixed shapes and volumes
Liquids: -fixed volumes but flow to assume the shape of their container
Gases: neither fixed shapes nor fixed volum
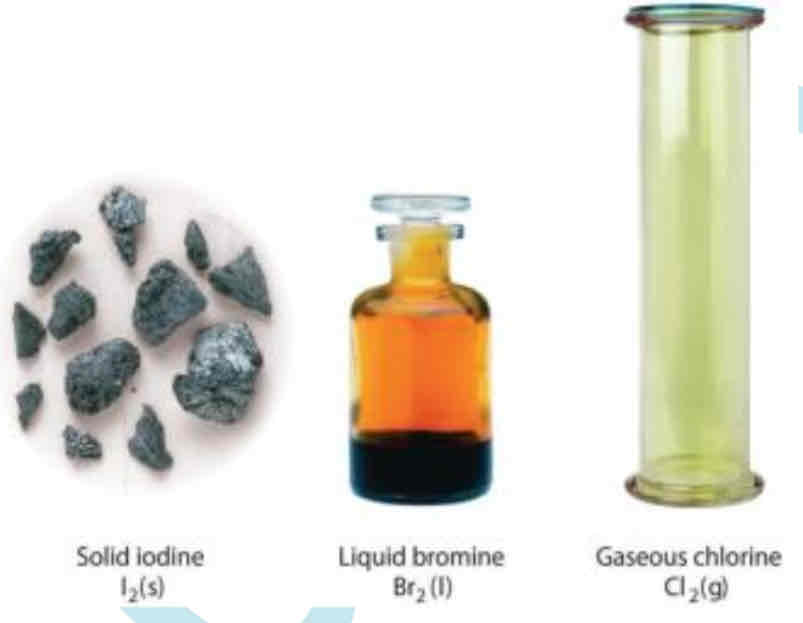
A pure chemical substance is?
And give me an example
It is any matter that has a fixed chemical composition and characteristics properties
Example it is oxygen and it is odorless ,colorless
What are the Types of Pure Substance?
And examples
There are two types
1- element : a substance that cannot be broken down into simpler particles
Ex : sodium
2- compounds:contains two or more elements bonded chemically
Ex : sodium chloride (table salt)
فصل لي ال matter وانواعه مع التعاريف
What is the physical property
physical properties: are characteristics that can be observed without changing the composition of sample
Example for physical property
Mass , colour , volume
What is the chemical properties?
describe the characteristic ability of a substance to react to form new substances
Example for chemical properties?
flammability and corrosion
What is the physical change?
are changes in which no bonds are broken or formed and Chemical composition of matter does not change
Ex for physical change
1-Changes of state (changes from a solid to a liquid or a gas and vice Versa)
2-Separation of a mixture
3-Physical deformation (cutting, denting, stretching)
4-Making solutions (special kinds of mixtures)
What is the chemical change?
occur when bonds are broken and/or formed Between molecules and atoms
Ex for chemical change?
Burning a candle
Rusting or Iron
Baking a cake
Electrolysis of water
What is the definition of density?
Density is defined as mass per unit volume
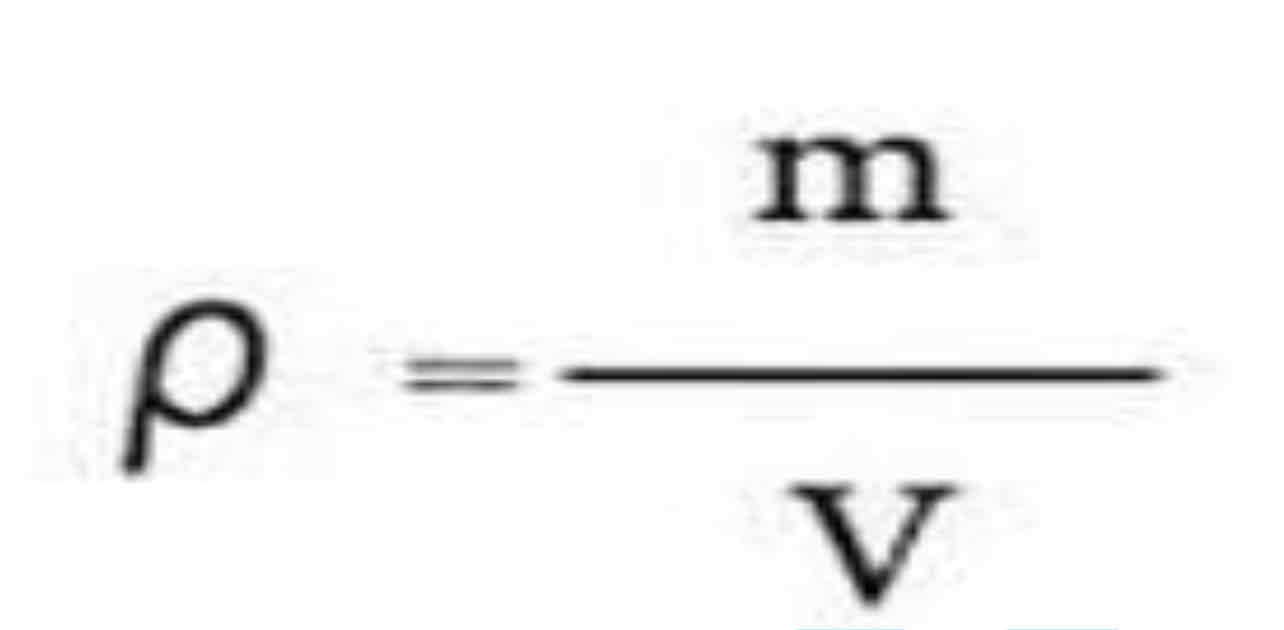
Density depends on
1- temperature
2- pressure
Density determines in lab by :
1-Dimensional method
2- Displacement method
determine the density of an unknow metal cube
that has side length 3.00 cm and mass is 310.672g
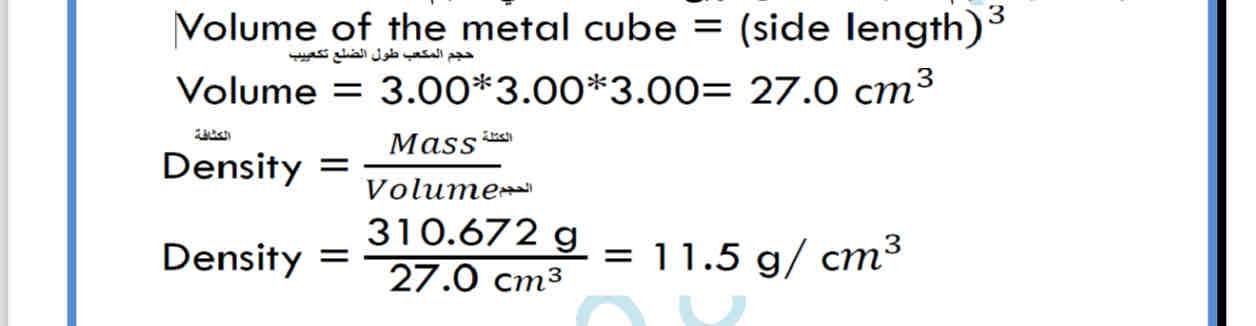
راجع ال significant figures بالكتاب
Nothing
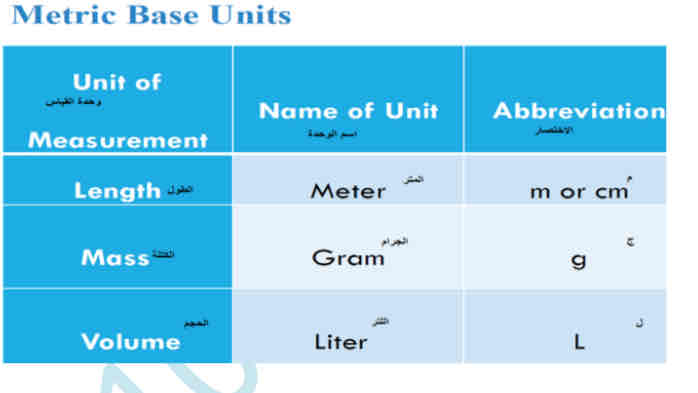
What is the metric system
Metric prefixes –are other units related to the base unit by power of 10.
What is postulates of Dalton’s atomic theory ?
1-An atom is the smallest unit of an element that can participate in a chemical change
2-an element consists of only type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element.
3. Atoms of one element differ in properties from atoms of all other elements.
4. A compound consists of atoms of two or more elements combined in a small, whole-number ratio. In a given compound, the numbers of atoms of each of its elements are always present in the same ratio.
5-Atoms are neither created nor destroyed during a chemical change
Modern atomic theory?
شكل الذرة وانها تحتوى p و n وحولها الكترونات
والقطر من النواة الى حدود الذرة 10 اوس -10
والقطر للنواة 10 اوس -15
What is the Atomic number and mass number? And what their symbols?
1. Atomic number (Z):is the number of protons in the nucleus of an atom
“Atomic number determines the identity of the atom”
Mass number(A)–is the of total number of protons and neutrons in an
A-Z =
No.Neutrons
Atoms are electrically neutral if
Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons.
When Atom called an ion ?
When the numbers of protons and electrons is not equal, the atom is
electrically charged and is called an ion.
What is the rule of chemical symbol?
Only the first letter of a symbol is capitalize
example: Co is symbol for element Cobalt
However, CO is compound carbon monoxide which has element Carbon and Oxygen in it
The symbol for an atom indicates the element via its usual two -letter
symbol, the mass number as a left superscript, the atomic number as a
left subscript (sometimes omitted), and the charge as a right superscript is called ?
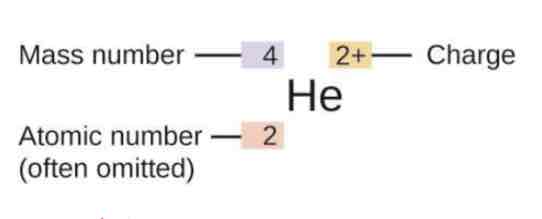
Isotopes are :
are nuclides having the same atomic number but different mass numbers
“Most elements occur in nature as mixtures of isotopes”
hydrogen are especially important
They have names and symbols

What is the isotopes effect?
when different isotopes exhibit different chemical behavior ,more common in lighter elements

