Chemistry - Atoms
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
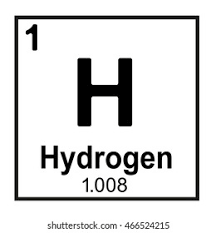
What does number 1 represent?
Atomic Number
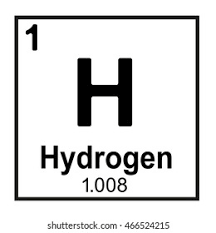
What does the letter “H” represent?
Chemical Symbol
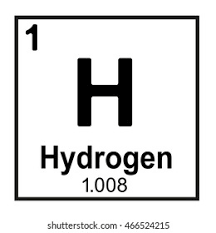
What does “Hydrogen” represent?
Name
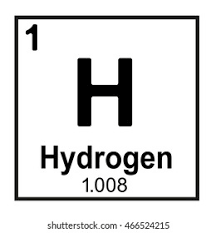
What does 1.008 represent?
Atomic Mass
Atomic Mass
The number of protons + the number of neutrons
Atomic Number
the number of protons OR the number of electrons in an neutral atom
Isotopes
atoms of the same element w/ different number of neutrons
What changes in isotopes
MASS and MASS ONLY

the “A”
Mass Number

the “Z”
Atomic Number

the “X”
Chemical Symbol
Average Atomic Mass
The average mass of all of the isotopes of an element
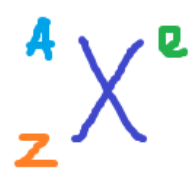
the “E”
Charge (electric charge)
You find the neutrons how?
Mass Number - Atomic Number
Element
substance made up of only one type of atom, each with the same number of protons.
Atom
The smallest part of a substance that cannot be broken down chemically
Isotope
An element where the atom have the same number of protons but w/ different number of nuetrons
Ion
is an atom or molecule that is charged due to a difference in number of electrons and protons.
Cation
Positive charged atom
Anion
Negative charged atom
Where is a proton located (inside or outside the nucleus)?
Inside
Where is a neutrons located (inside or outside the nucleus)?
Inside
Where is a electron located (inside or outside the nucleus)?
Outside
Protons charge
Positive
Neutrons charge
Neutral
Electrons Charge
Negative
Protons relative charge?
1
Neutrons relative charge?
1
Electrons relative charge?
Negligible
What’s the atomic number determined by?
Protons
What’s the mass number determined by?
Neutrons and Protons
What’s the charge determined by?
protons - electrons
What does the atomic number identify?
Element
What does the mass number identify?
The mass
What does the charge identify?
atom (charge = 0; p = e)
ion (charge doesn’t = 0; p doesn’t = e)
400bc
Democritus
Atomos
Shape = Behavior
200bc
Aristotle
4 Elements
1789
Lavosier
Law of Conservation of Mass
1803
John Dalton
Solid Sphere
Everything’s made of indestructive spheres
1897
JJ Thompson
Plum Pudding
Showed atoms have smaller parts
1911
Ernest Rutherford
Alpha Beta Gamma Rays
Gold-Foil Experiment
1912
Henry Mosely
Atomic Mass
1913
Neil Bohr
Planetary
Everythings a circle w/ a positive center
1926
Erwin Schrodinger
Cloud of Electrons