Biology - Chapter 20: Gene Expression
1/70
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
71 Terms
6 types of mutations
Substitution, addition, deletion, inversion, duplication and translocation
3 possible consequences of a substitution mutation
A stop codon forms and production of the polypeptide is stopped prematurely
A different codon forms, which codes for a different amino acid
A different codon forms, which codes for the same amino acid
When might an addition mutation have less of an effect? Why?
When 3 bases (or a multiple of 3 bases) are added as there will be no frameshift
Cell differentiation
The process by which each cell develops into a specialised structure suited to the role that it will carry out
Where are all the cells in an organism derived from?
A zygote
Why can only certain types of cells produce certain proteins?
Although all cells contain all genes, only certain genes are expressed in any one cell at any one time
Totipotent cells
Cells that can divide and produce any type of body cell
Why don’t cells make all the proteins possible?
It would waste energy and resources
2 ways genes are prevented from being expressed
Preventing transcription (i.e. the production of mRNA)
Preventing translation
Stem cells
Cells that have the ability to differentiate into other cells
4 sources of stem cells
Embryonic stem cells
Umbilical cord blood stem cells
Placental stem cells
Adult stem cells
4 types of stem cells
Totipotent
Pluripotent
Multipotent
Unipotent
2 examples of totipotent stem cells
Early embryos and zygotes
Pluripotent stem cells
Cells that can differentiate into almost any type of cell
2 examples of pluripotent stem cells
Embryonic stem cells and fetal stem cells
Multipotent stem cells
Cells that can differentiate into a limited number of specialised cells
2 examples of multipotent stem cells
Adult stem cells and umbilical cord stem cells
Unipotent stem cells
Cells that can only differentiate into a single type of cell
Where are unipotent stem cells derived from?
Multipotent stem cells
Induced pluripotent stem cells
A type of pluripotent stem cell that is produced from somatic cells
iPS
Induced pluripotent stem cell
Why are iPS special?
They are capable of self-renewal, so could potentially divide indefinitely to provide a limitless supply
What might pluripotent stem cells be used for?
To regrow tissues that have been damaged
Transcriptional factors
Molecules that switch genes on so that transcription of the gene can begin
How do transcriptional factors affect transcription? (5)
The transcriptional factor has a site that binds to a specific base sequence of DNA in the nucleus
When it binds, it causes this part of the DNA base sequence to begin transcription
mRNA is produced and it is used to produce a polypeptide
If the gene is not being expressed, the site of the transcriptional factor complimentary to the DNA base sequence is not active
This means it cannot cause transcription and protein synthesis
How does oestrogen switch on genes? (5)
It diffuses across the phospholipid bilayer
It binds to the complementary receptor on a transcriptional factor
This activates the transcriptional factor as its DNA binding site is now complimentary to and can bind to DNA
The transcriptional factor enters the nucleus and binds to the specific base sequence on DNA
This stimulates transcription of the gene
Epigenetics
Heritable changes in gene function without changing the base sequence of DNA
Epigenome
A layer of chemical tags that cover DNA and histones
What does the epigenome do?
It determines the shape of the DNA-histone complex, which affects what genes are switched on or off
Epigenetic silencing
When the epigenome keeps inactive genes in a tightly-packed arrangement, ensuring that they cannot be read
Is the epigenome fixed or flexible? Why?
Flexible - its chemical tags respond to environmental changes
Somatic cells
Cells in the body other than the sperm and egg cells
What might environmental signals cause proteins to change? (2)
The acylation of histones, leading to the activation or inhibition of a gene
The methylation of DNA by attracting enzymes that can add or remove methyl groups
What happens when the association of histones with DNA is strong? (3)
The DNA-histone complex is more condensed
The DNA is not accessible by transcription factors, so cannot initiate the production of mRNA
The gene is switched off
What happens when the association of histones with DNA is weak? (3)
The DNA-histone complex is less condensed
The DNA is accessible by transcription factors, so can initiate the production of mRNA
The gene is switched on
What happens as a result of the decreased acetylation of associated histones? (4)
Fewer acetyl groups bond to the R groups in histones, so the positive charges on histones remain (so increase)
This increases their attraction to the phosphate groups of DNA
This makes the association between DNA and histones stronger, so the DNA is not accessible to transcription factors
mRNA production is not initiated - the gene is switched off
Acetylation
The process whereby an acetyl group is transferred to a molecule
Deacetylation
The process by which an acetyl group is removed from a molecule
What group donates the acetyl group in acetylation?
Acetylcoenzyme-A
How does the increased methylation of DNA inhibit the transcription of genes? (2)
It prevents the binding of transcriptional factors to DNA
It attracts proteins that condense the DNA-histone complex (by inducing the deacetylation of histones), making the DNA inaccessible to transcription factors
Methylation
The addition of a methyl group to a molecule
In methylation, what is the methyl group added to?
The cytosine bases of DNA
What is oestrogen?
A steroid hormone
What happens when acetyl groups are added to DNA-histone complexes? (3)
They bind to the R groups of the histones, removing the positive charge on NH3+. This reduces the attraction between the phosphate backbone and the R group, so DNA loosens from the histone.
Give three characteristic features of stem cells.
Undifferentiated cells
Can differentiate into other cells
Can keep dividing
What is the effect of epigenetic changes on mutations? Why?
They can increase the incidence of mutations e.g. if they cause an increase in the methylation of protective genes so damaged DNA base sequences are not repaired and can lead to cancer
How might epigenetic therapy be used to treat diseases? (2)
Drugs may be designed that inhibit enzymes involved in acetylation or methylation (must be specific to cancer cells)
They can be used to develop diagnostic tests that can detect the early stages of diseases
How does siRNA affect gene expression? (5)
Enzyme cuts large double-stranded RNA into siRNA
One of the two strands combines with an enzyme
siRNA molecule guides the enzyme to an mRNA molecule by pairing up its bases with the complementary section on mRNA
Enzyme cuts mRNA into smaller strands
mRNA can no longer be translated into a polypeptide - gene has not been expressed
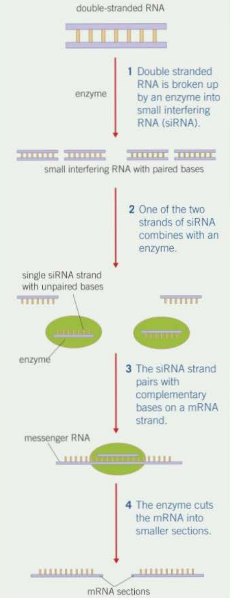
siRNA
Small Interfering RNA
Cancer
A group of diseases caused by damage to the genes that regulate mitosis and the cell cycle
2 types of tumours:
Malignant (cancerous) and benign (non-cancerous)
Benign tumours vs malignant tumours (9)
Similarities:
Both grow to a large size
Differences:
Grow very slowly vs rapidly
Relatively normal nucleus vs larger and darker (abundance of DNA)
Specialised cells vs unspecialised cells
Cells stick together and remain within tissue - primary tumours (due to adhesion molecules produced) vs Cells spread to other regions of the body by metastasis, don’t produce adhesion molecules - form secondary tumours
Tumours surrounded by capsule so compact structure vs No capsule so tumours can grow finger-like projections into surrounding tissue
Less likely to be life-threatening but may affect functioning of vital organs vs More likely to be life-threatening (replacement of normal tissue by abnormal)
Localised effects on body vs Systemic effects
Usually removed by surgery only vs Radiotherapy, chemotherapy and/or surgery
Rarely reoccur after treatment vs more frequently reoccur after treatment
2 types of genes involved in cancer
Oncogenes and tumour suppressor genes
How do oncogenes form?
As a result of mutations to proto-oncogenes
What happens if proto-oncogenes are mutated to form oncogenes? (1+2+1)
It becomes permanently activated so:
Receptor protein on CSM can be permanently activated so cell division is switched on, even without growth factors
Oncogene may code for a growth factor that is then produced in excessive amounts, stimulating cell division
Cells divide too rapidly and a tumour or cancer develops
What do tumour suppressor genes do? (3)
Slow down cell division
Repair mistakes in DNA
Tell cells when to die / undergo apoptosis
What might happen if a tumour suppressor gene is inactivated?
The mutation makes the gene become inactive
It stops inhibiting cell division, so cells divide uncontrollably
How can the abnormal methylation of tumour suppressor genes result in the development of tumours? (4)
Increased methylation
The tumour suppressor gene is not transcribed and is inactive
Proteins that cause cells to undergo apoptosis and that repair damaged DNA are not produced - these processes do not occur
This results in uncontrollable cell division and the formation of a tumour
How can the abnormal methylation of oncogenes result in the development of tumours? (3)
Decreased methylation means the oncogene is transcribed more and is active
This means cell division takes place at an increased rate
This results in the formation of a tumour
How can increased oestrogen concentrations affect the development of some breast cancers? (6)
After menopause, the fat cells of the breast tissue produce more oestrogen
This could cause a gene that promotes transcription to be switched on, so more transcription and more cell division
This causes a tumour to develop
The tumour then also increases oestrogen concentration
WBCs are drawn to the tumour, which also causes an increase in oestrogen concentration
This causes the tumour to develop more
Bioinformatics
The science of collecting and analysing complex biological data
Why was it possible to sequence the entire genome so quickly?
Bioinformatics - the use of computers to read, store and organise biological data at a faster rate than before as well as to analyse and interpret the data
How is the complete DNA base sequence of an organism determined?
By using whole-genome shotgun sequencing - DNA is cut into many small, easily sequenced sections, then computer algorithms are used to align overlapping segments to assemble the entire genome
Why can genomes be sequenced more rapidly as time goes on?
Because sequencing methods are continuously updated and the processes involved are becoming increasingly automated
Why is it easier to determine the proteome of prokaryotic organisms from the genome compared to the proteome of eukaryotic organisms? (2)
The vast majority of prokaryotes have just one circular piece of DNA not associated with histones
There are no non-coding portions of DNA, unlike in eukaryotic cells
How might the knowledge of the genome of an organism be applied to produce vaccines against pathogens?
The proteome of an organism could be identified, which could then be used to identify potential antigens for use in vaccine production
In more complex organisms, why might it be harder for knowledge of the genome to be translated to the proteome?
Because of the presence of non-coding DNA and regulatory proteins
What must happen for a gene to be transcribed more? (2)
Transcription factors will bind to promoter region
RNA polymerase will be stimulated
When testing the effect of a new drug (e.g. on cancer), why would you give the control group the older drug?
it would not be ethical to fail to treat their disease (e.g. cancer)
How might single-stranded DNA molecules be used to reduce concentrations of a protein? (3)
DNA is complementary to the mRNA that codes for the protein
The DNA binds to the mRNA
This prevents translation
How does siRNA work? (Exam q, 2)
siRNA destroys mRNA
This prevents the mRNA from being translated