Chemistry: Electron Configuration and Nuclear Decay
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Aufbau Principle
electrons enter energy levels around the nucleus from lowest energy level to highest energy level
Pauli Exclusion Principle
Orbitales can occupy, at the most, only 2 electrons in opposite spin
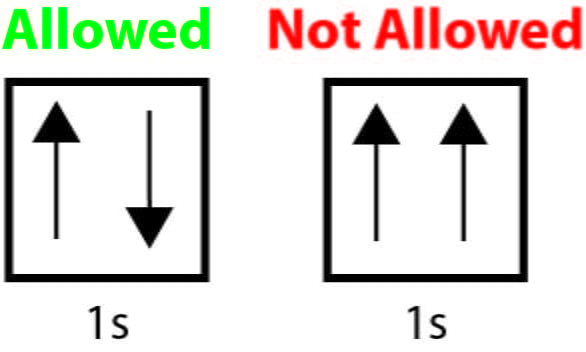
Hund’s rule
in orbitales of equal energy, electrons enter singly first before they pair up
“up up up” before “down down down”
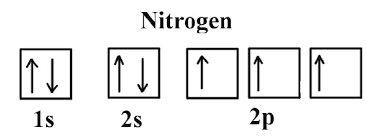
types of radiation
alpha, beta, gamma

alpha particle
Charge: +2
Stopped by: paper, thin clothing, skin
nuclear decay giving off a helium nucleus
least penetrating nuclear decay
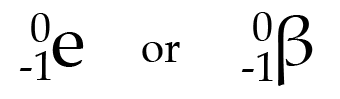
beta particle
Charge: -1
Stopped by: books, thick clothing, foil (aluminum)
decay emitting an electron
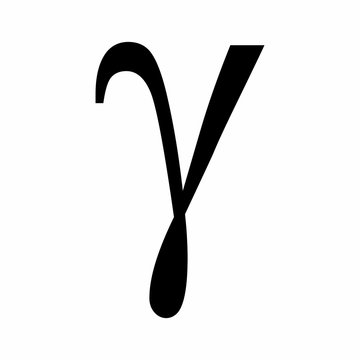
gamma particle
charge: 0
stopped by: concrete, thick lead
nuclear decay with no mass and no charge
most damaging nuclear decay to human body