acids bases and salts
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
What do metals turn to when reacted with acid?
A salt + hydrogen
What do oxides of metals turn to when reacted with acid?
A salt + water
What do hydroxides of metals turn to when reacted with acid?
A salt + water
What do carbonates turn to when reacted with acid?
A salt + water + carbon dioxide
What do hydrogencarbonates turn to when reacted with acid?
A salt + water + carbon dioxide
What do ammonia turn to when reacted with acid?
An ammonium salt
What do acids release in aqueous solutions
H+ ions
What does monoprotic mean
An acid that only donates 1 proton (H+ ion) per molecule
give an example of a monoprotic molecule
Hydrochloric acid ( H+ + Cl-)
What does dirprotic mean
the acid donates 2 protons (H+ ions)
give an example of a diprotic molecule
Sulphuric acid
What is the formula of a carbonate ion
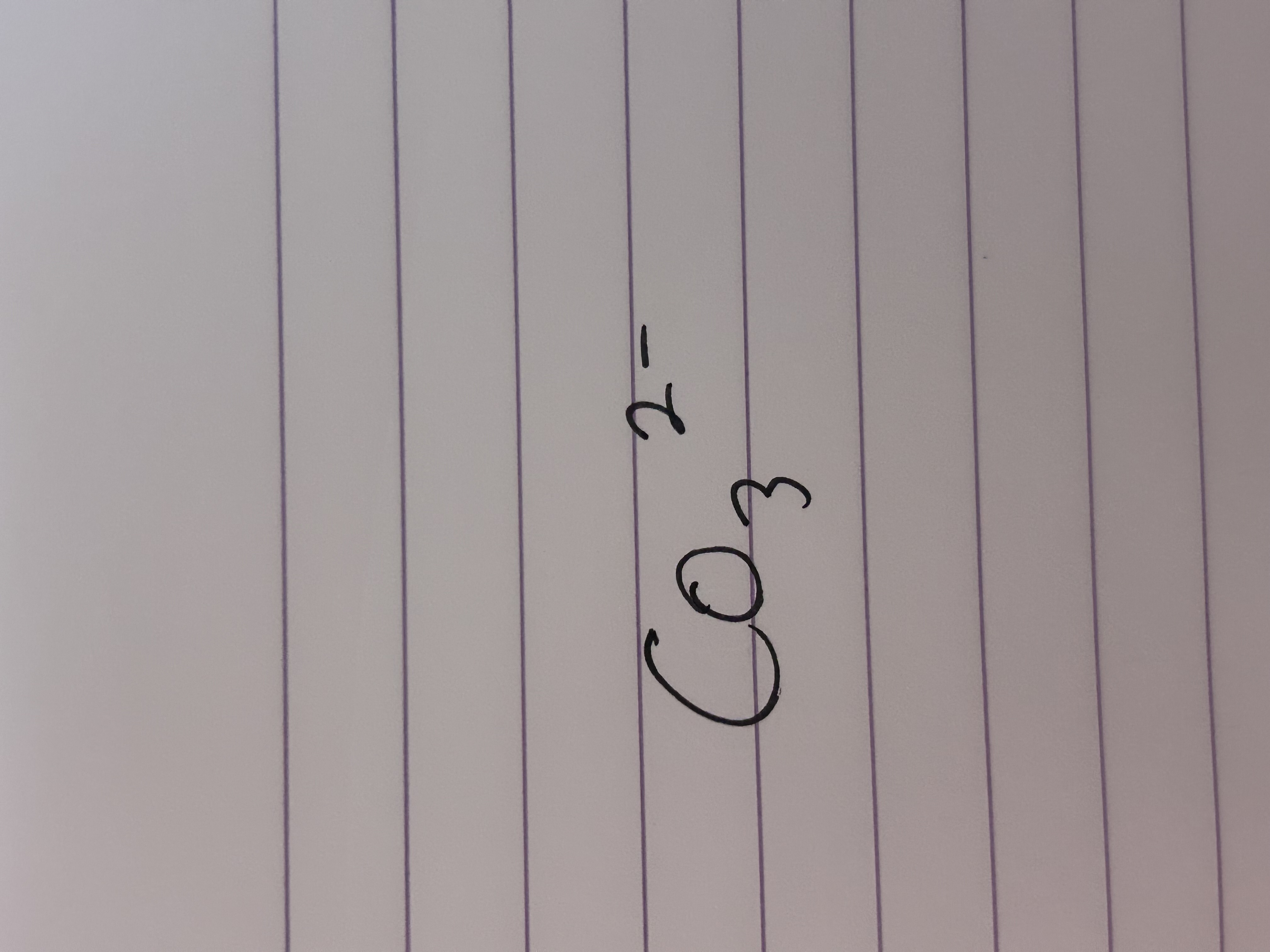
What formulae of ammonia
NH3
What is alkali
Soluble bases which OH- ions in aqueous solutions
What does hydrochloric acids make
Chlorides
What does nitric acid make
Nitrites
What does sulphuric acid make
Sulphates or hydrogensulphates
What is the formula of nitric acid
HNO3
What is sulphuric acid
H2SO4
What is the formula of ammonium
NH4+
hydroxide formula
OH-
Nitrate formula
NO3-
Nitrite formula
NO2-
Hydro carbonate formula
HC03-
Manga are (VII) (permanaganate) formula
MnO 4-
sulfate formula
SO4 2-
Sulfite formula
SO3 2-
Dichromate (VI) formula
Cr2O7 2-
Phosphate formula
PO4 3-
What’s binary compound
A binary compound contains 2 elements only
What colour does hack turn litmus paper
Red
What do the Roman numerals mean after transition metal
The charge. (E.g. iron (II) is 2+