Lecture 10: Development of T Lymphocytes
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
What is lymphopoiesis?
production of new lymphocytes in central lymphoid organs
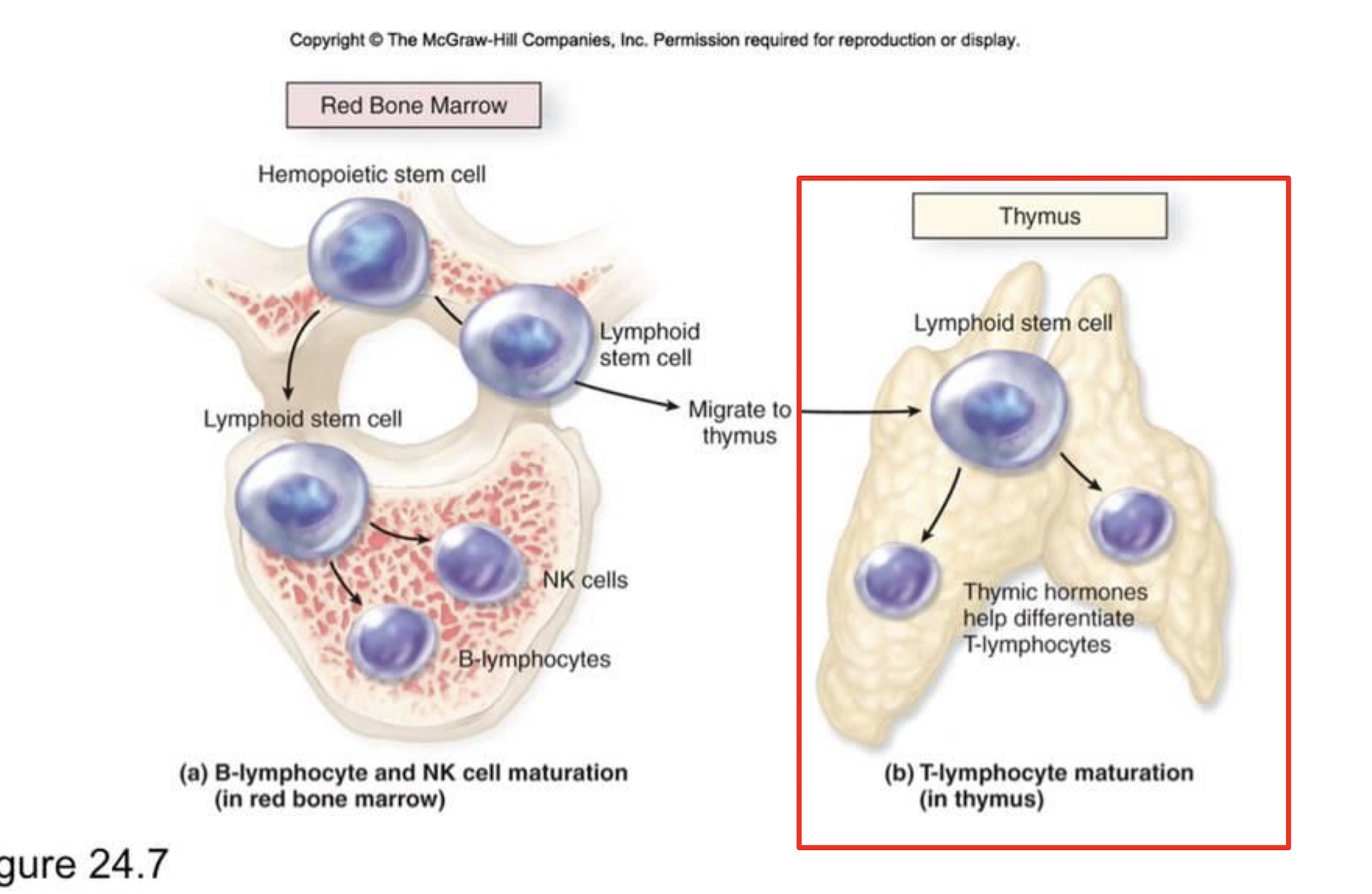
When do T cells move to the thymus?
common lymphoid progenitors travel in the blood as immature T cell precursors and then go as thymocytes to the thymus where it develops T cell specific markers and undergoes thymus selection and education
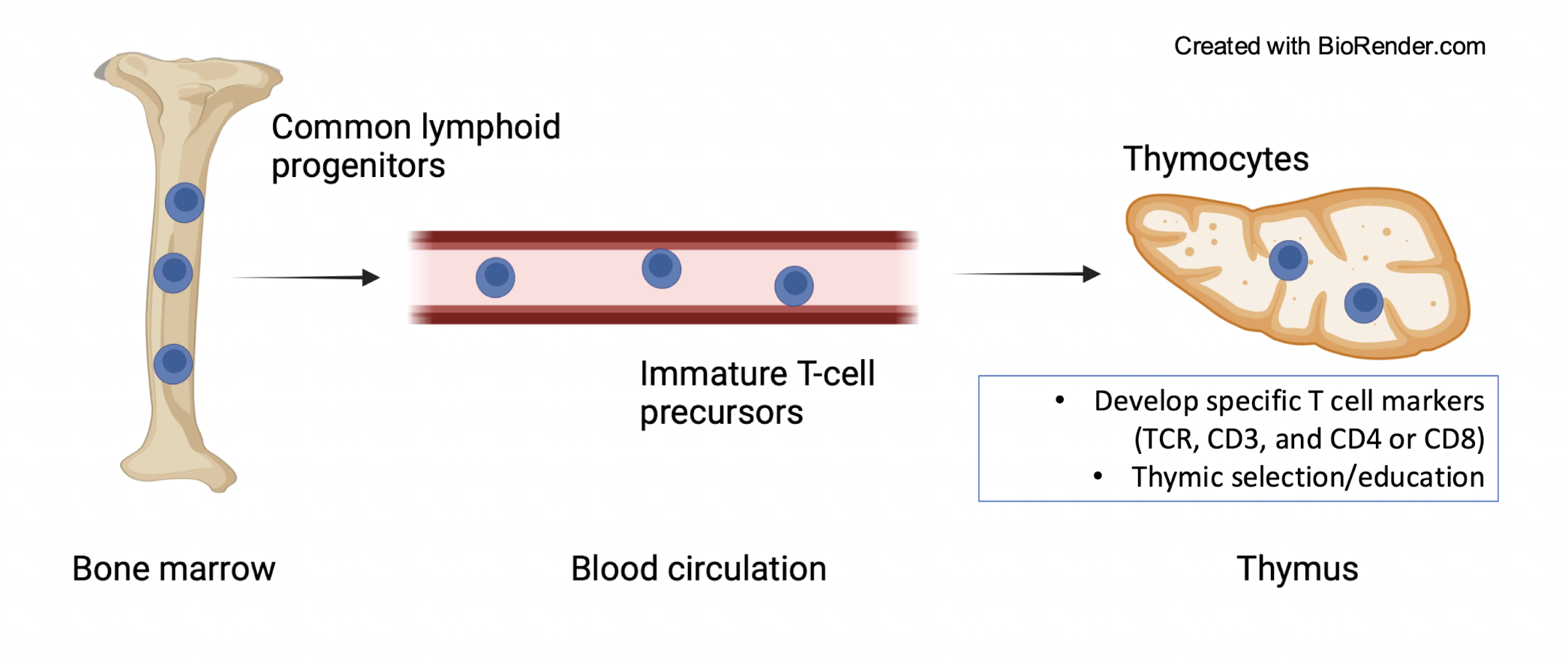
What are the specific T cell markers?
TCR, CD3 and CD4 or CD8
What is the structure of the thymus?
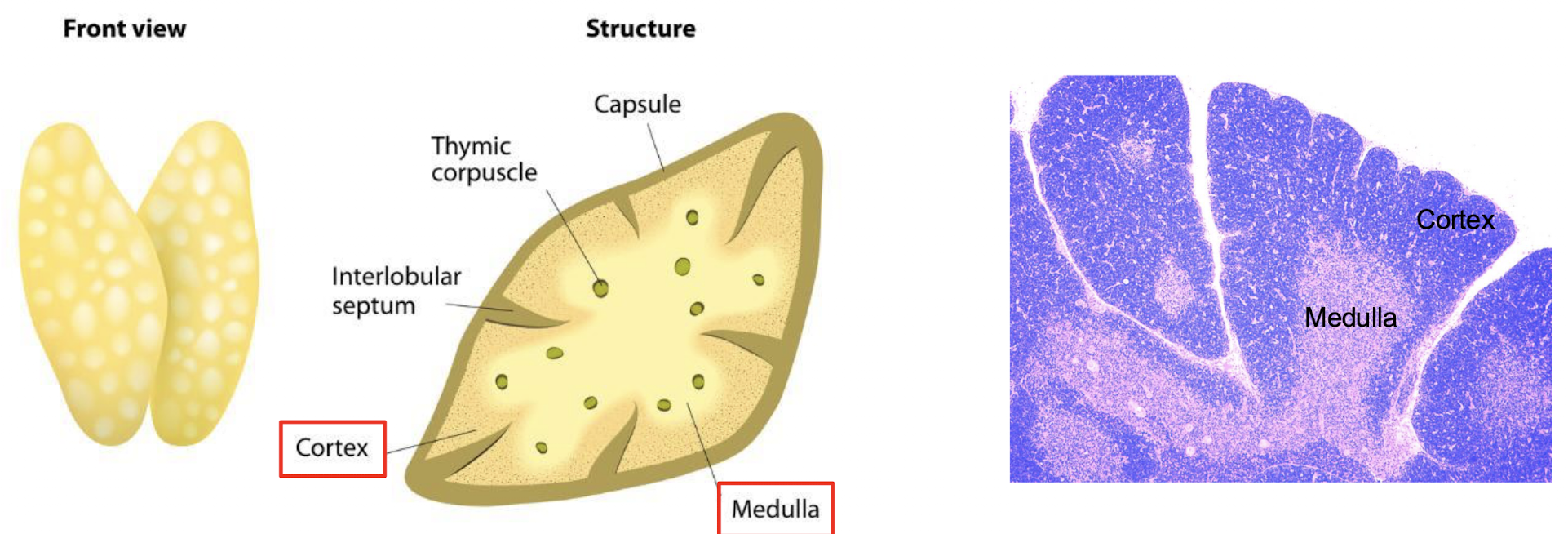
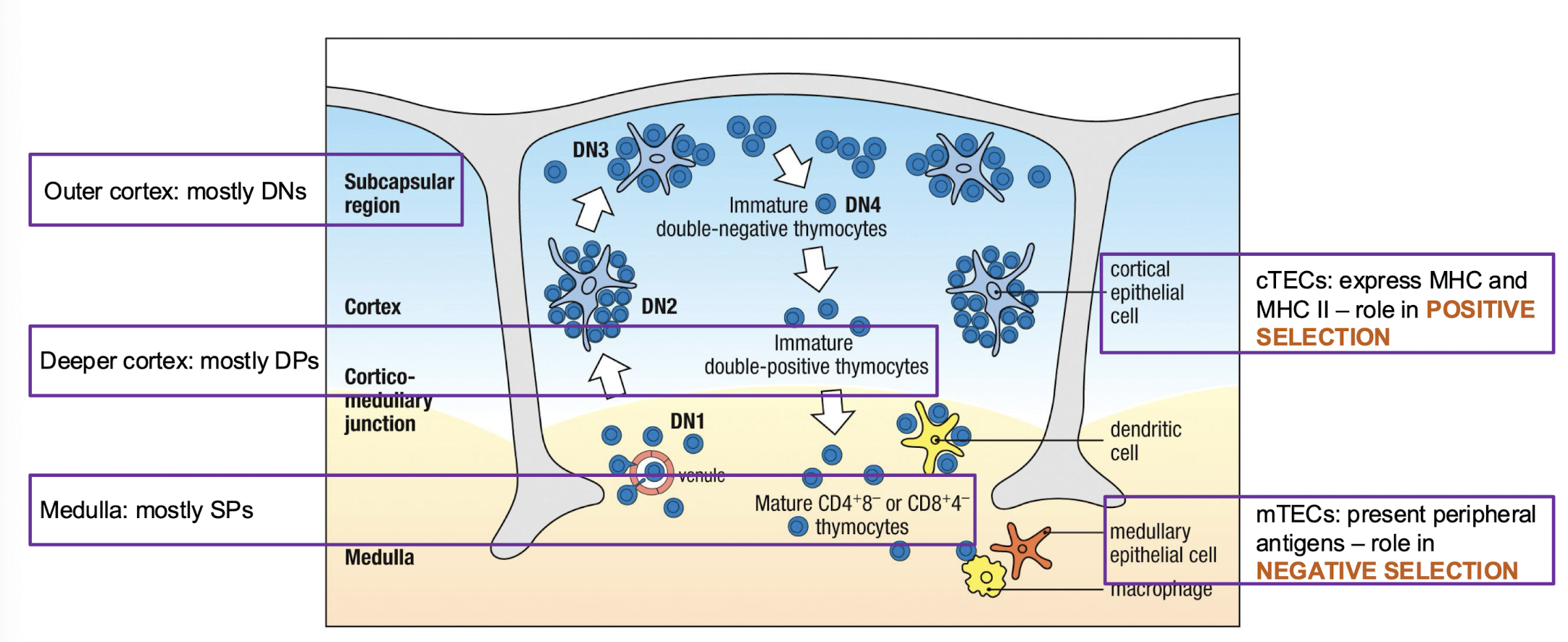
How are cells organized in the thymus?
Cortex:
immate thymocytes
Scattered macrophages
Medulla:
more mature thymocytes
macrophages
dendritic cells
few B cells
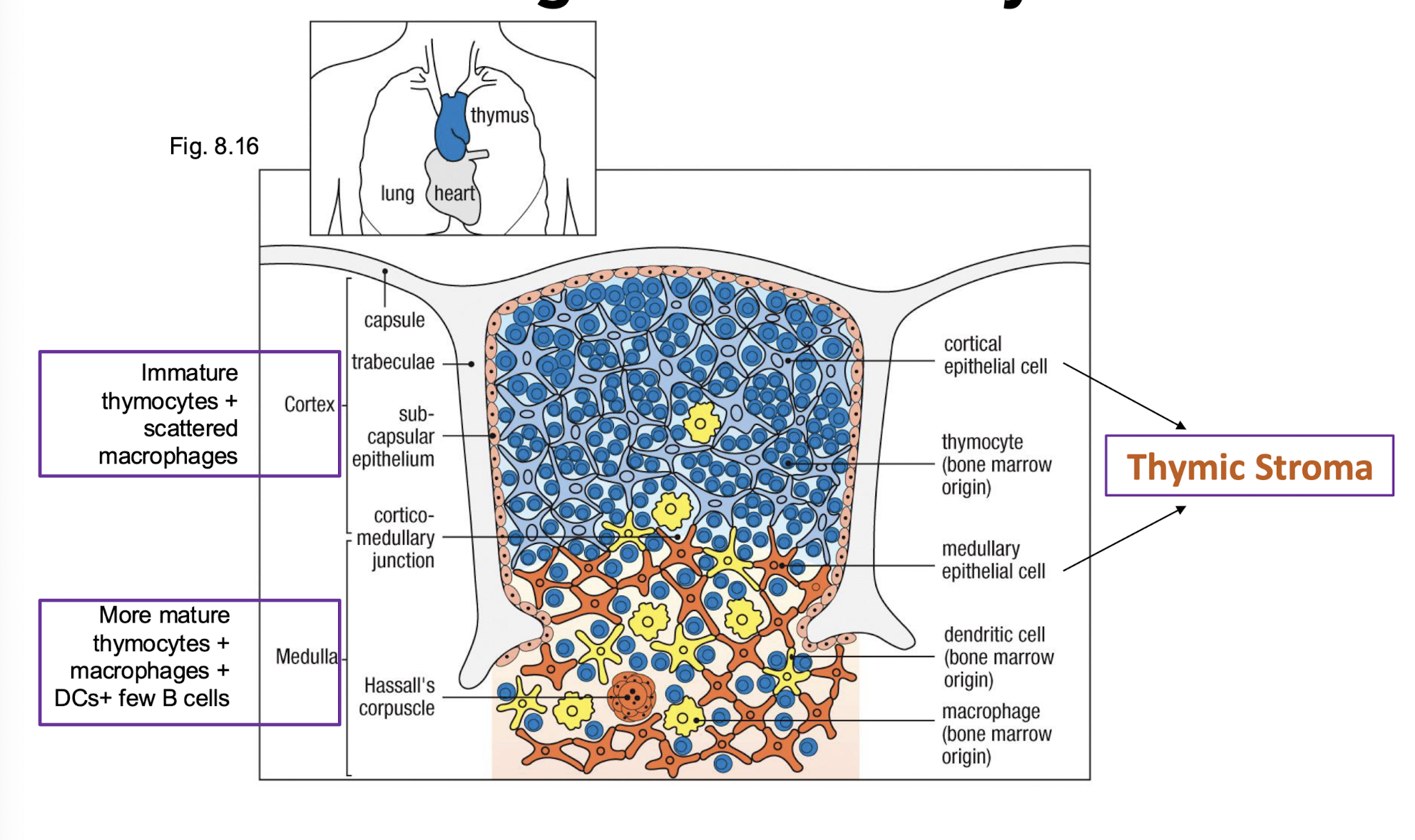
What makes up the thymic storma?
stomal cells → structural support and the development of thymocytes → cortical epithelial cells and medullary epithelial cells are the stromal cells
What happens to T cell precursors in the thymus?
T cell precursor proliferate extensively in the thymus → but most die there
the key player in apoptosis → macrophages in the thymus cortex and medulla
What does the thymus contain in a young adult mouse
10^8 thymocytes
10^7 generated everyday
only 10^6 leave the thymus each day (2-4%)
98% die by apoptosis
What is self tolerance?
each thymocyte carefully tested to make sure it can recognize shelf MHC with self peptides and that it wont react strongly against the body own tissues
if there is any chance it will react →die
the immune system's ability to recognize its own cells and tissues as non-threats and not attack them
What are thymocytes?
T cell undergoing development in the thymus → no education or selection has taken place
What are the thymocyte lineages?
gamma-delta T cells
alpha-beta T cells → develop into CD4 and CD8 → majority
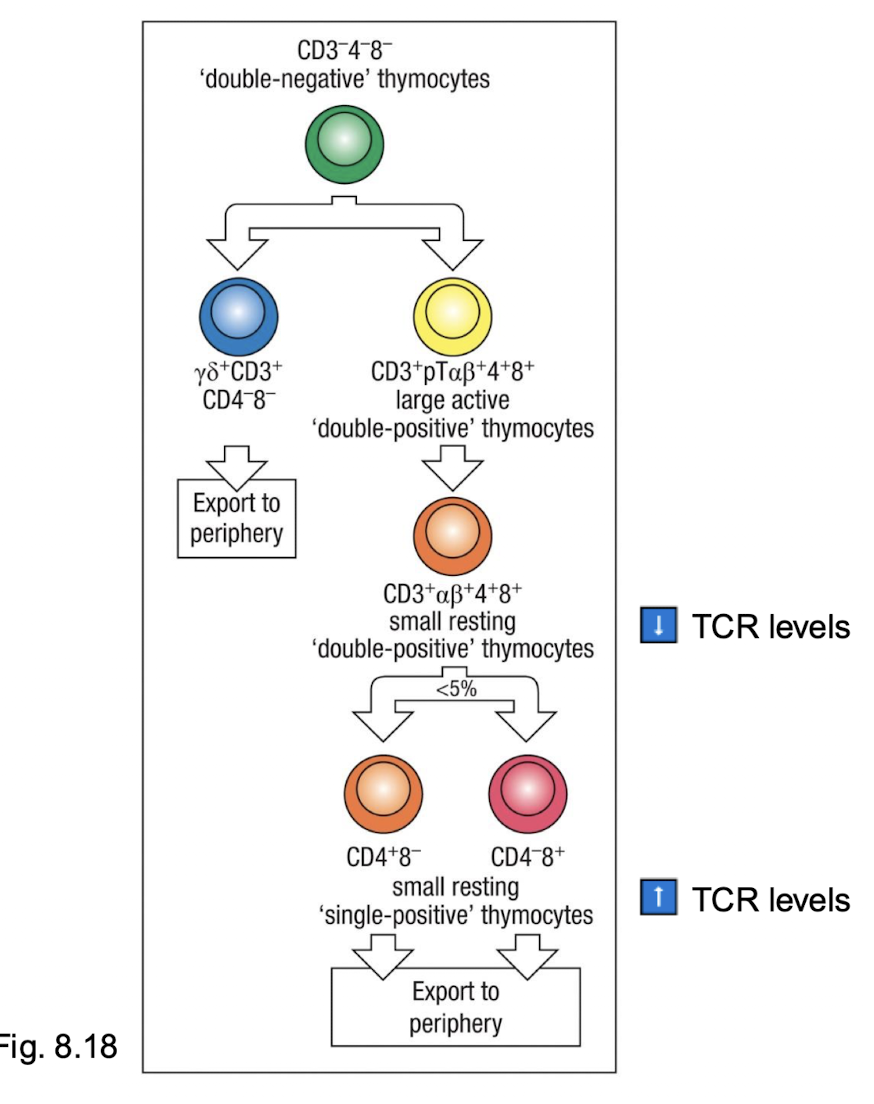
What marks the stages of thymocyte development?
status of the TCR genes
changes in TCR expression
changes in cell surface molecules expression (CD3, CD4 and CD8)
different combinations of cell surface proteins used as markers for T cells at different stages of differentiation
How are these two lineages created?
Double negative means no 4 and No 8 → then double positive with 4 and 8 → then selection makes single positive with either 4 or 8 and only single leaves the thymus
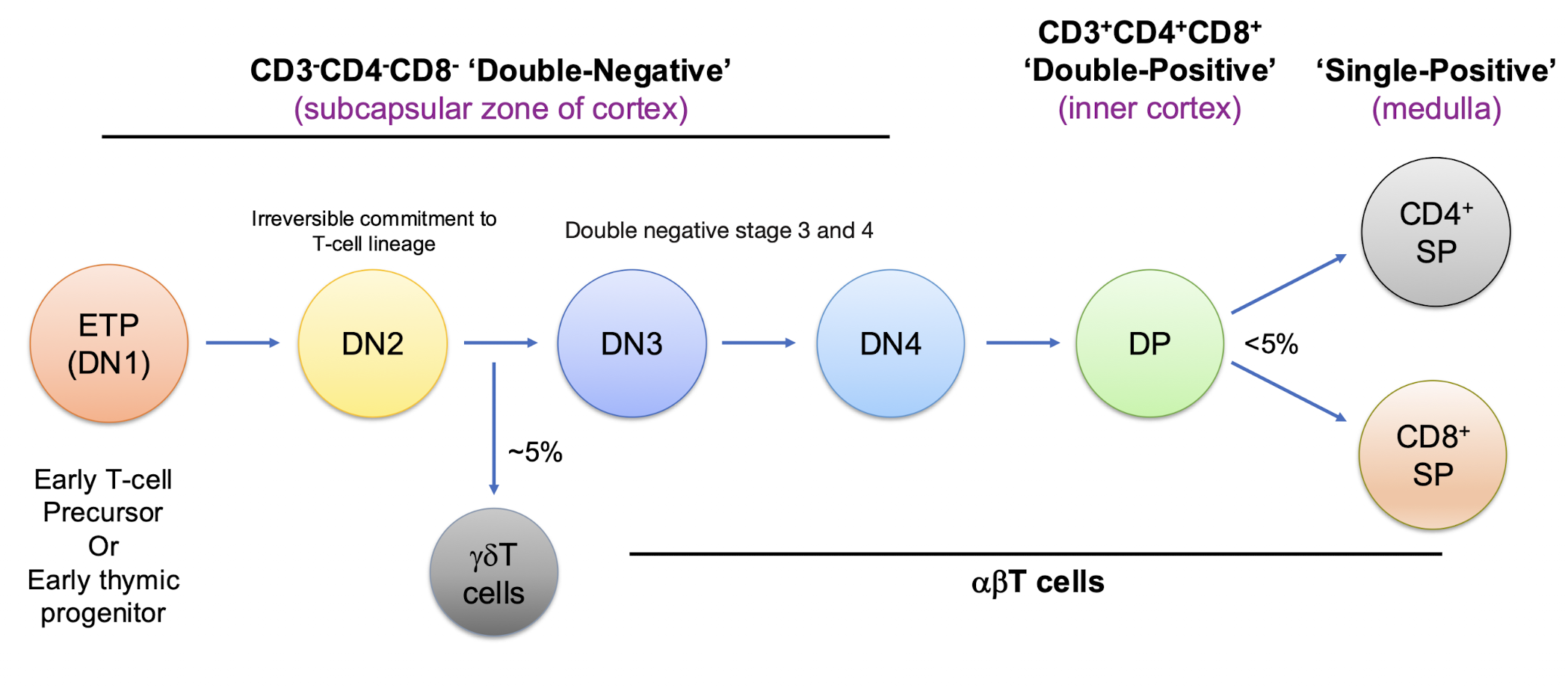
When are ETP’s committed to becoming T cells?
when it becomes a double negative
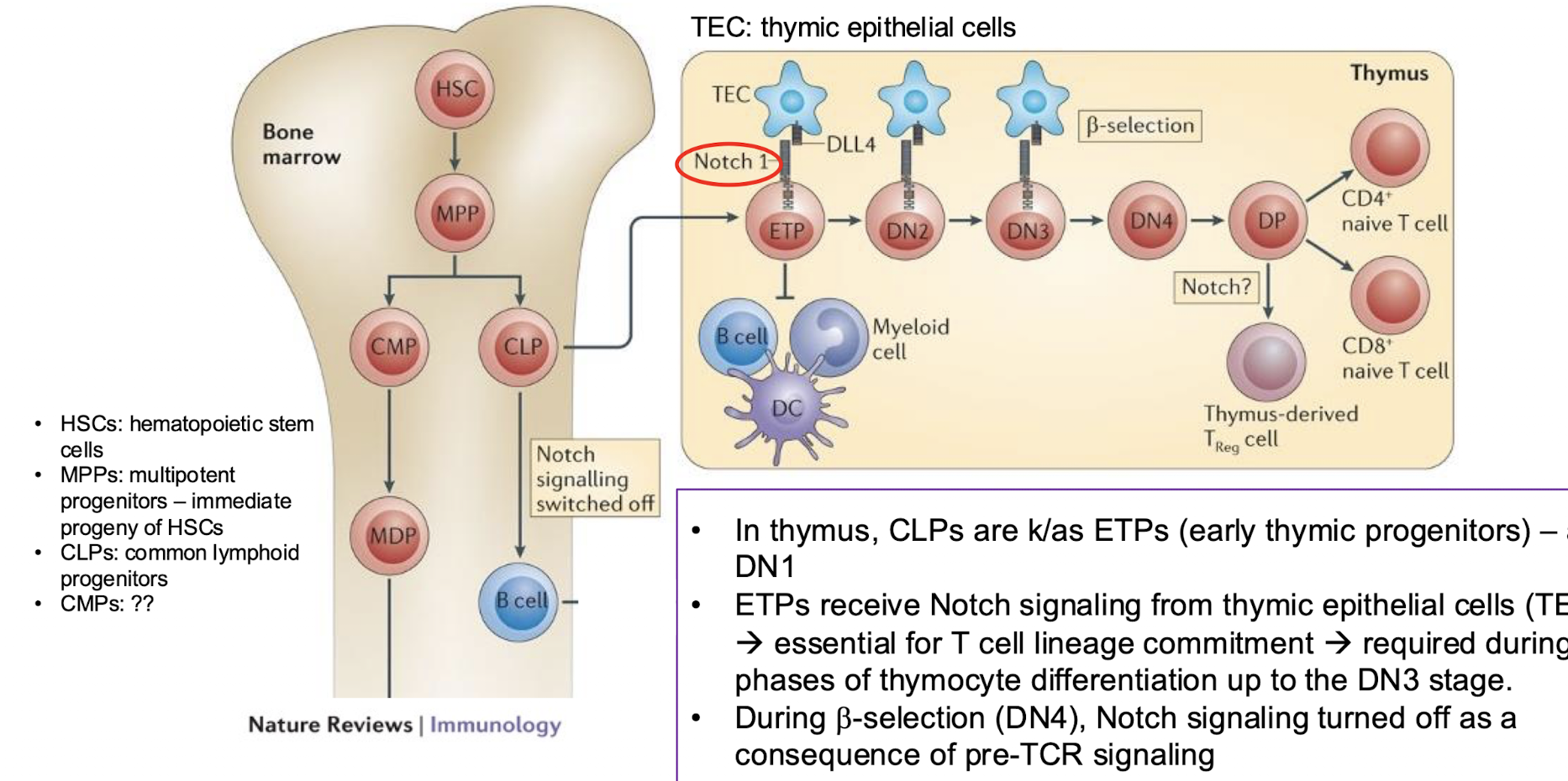
What is notch signalling?
commitment to T cell lineage occurs in the thymus after Notch signalling → tells to become a T cell from a common lymphoid progenitor
not present in the bone marrow which is why they go to the thymus
How does notch signalling occur?
in thymus, CLPs are K/as ETPS (early thyme progenitors) → aka DN1
ETPS receive notch signalling from thyme epithelial cells (TECs) → essential for T cell lineage commitment → required during early paces of thymocyte differentiation up to DN3
What are the earliest T cells precursors in the thymus?
have not rearranged TCR loci
do not expression CD4 or CD8
called double negatives
Markers → c-KIT, CD44, CD25
What is c-KIT
receptor for stem cell growth factor
What is CD44?
adhesion molecule → homing to thymus
What is Cd25
alpha chain of IL2 receptor
What are the names of the double negative stages?
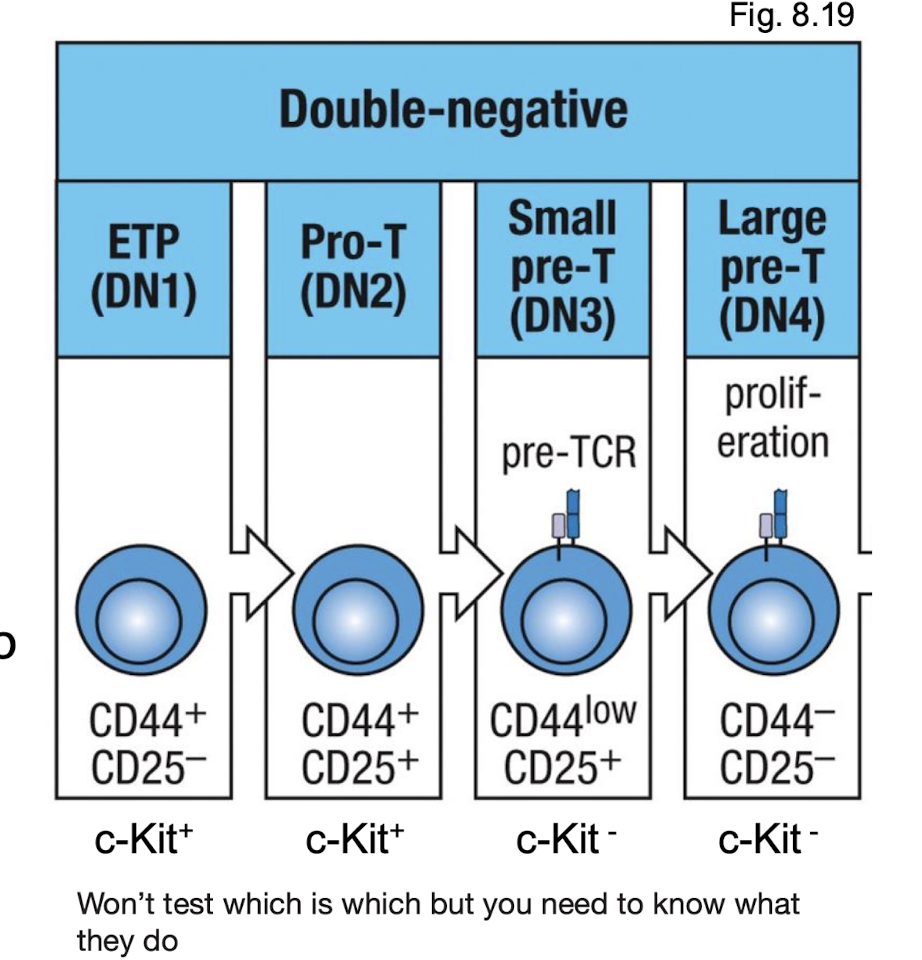
When is the pre TCR formed?
DN3
What does CD3 expression begin?
between DN2 and DN3→ small fraction of DN2 matures into gamma delta TCR while most proceed to alpha beta TCR
What does the beta chain rearrangement start?
DN2
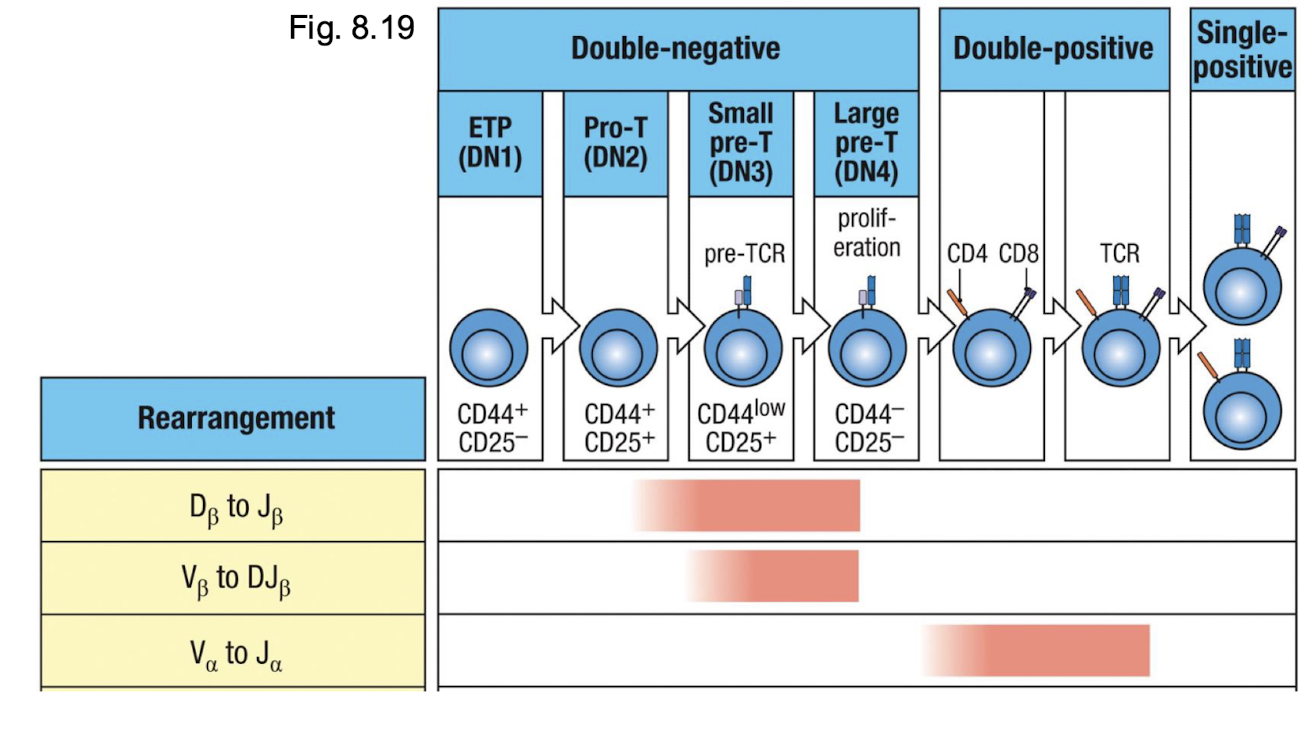
How is the pre TCR formed?
in DN3, newly formed beta chain combines with the pre T alpha (surrogate chain) and CD3 to form the pre TCR → the real alpha chain has not formed yet
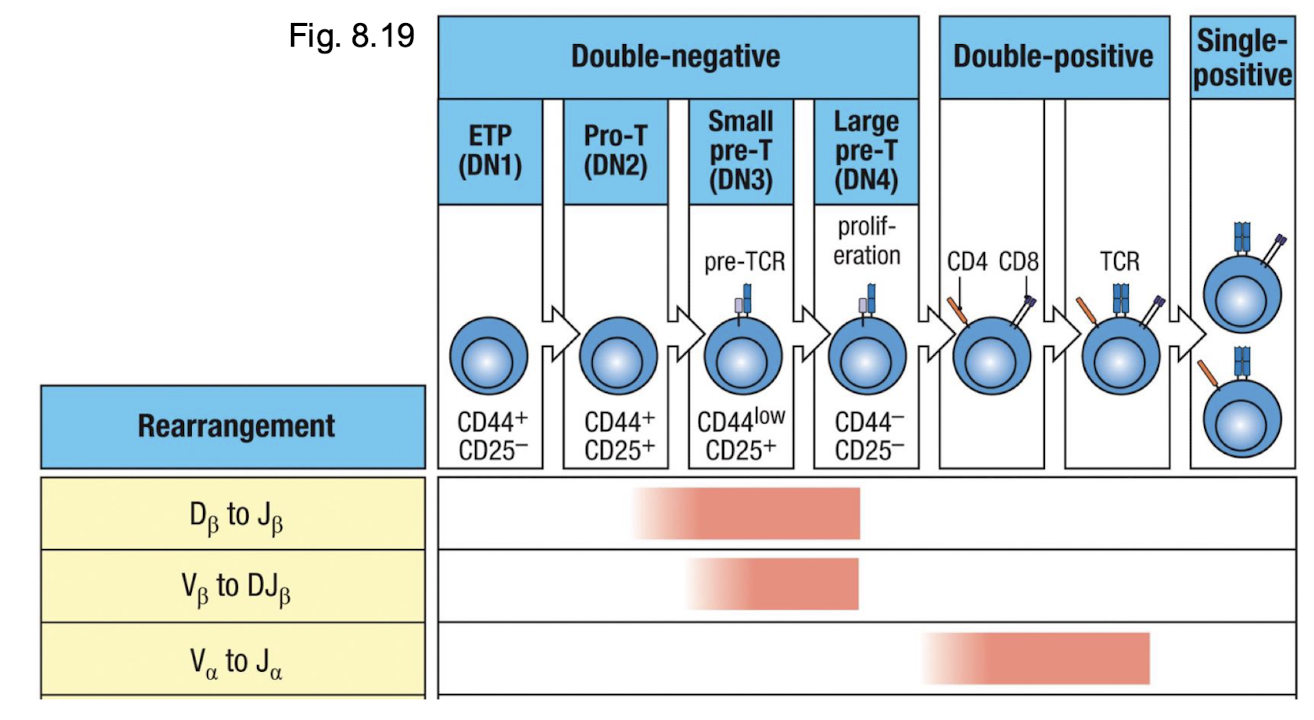
What happens if the B chain arrangement is productive?
proceed to DN4 → CD4 and CD8 expression is initiatred
What happens once it is a double positive?
DP begins to rearrange alpha chan locus → low levels of alpha beta TCR and CD3 → ready for selection
heat map of T cell development
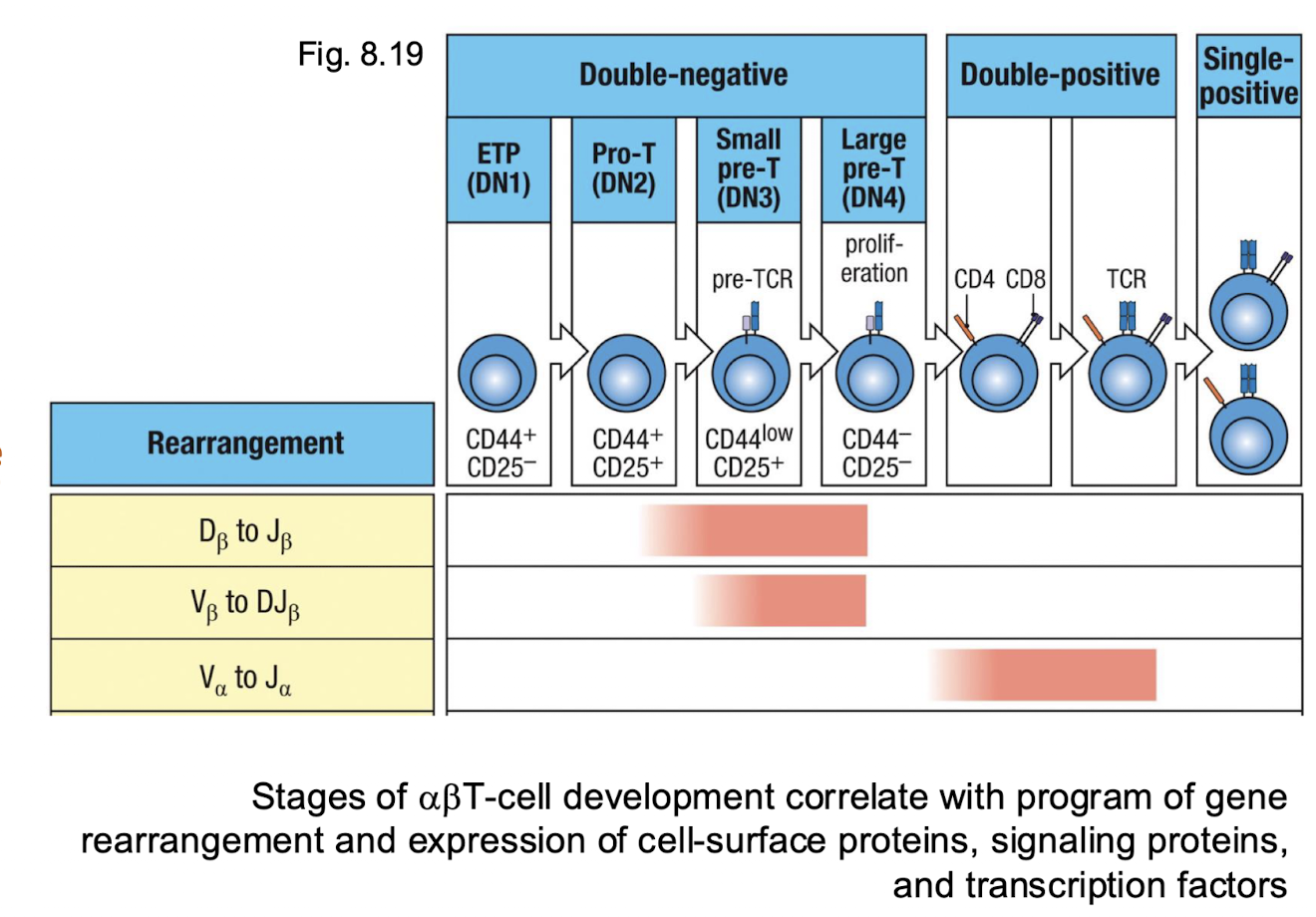
When is the NOTCH expressed?
tells the cell to become the T cell
expressed in early DN1-DN3
commitment signal. → directs progenitor entering thymus to become T cell instead of B or NK cells
without → thymocytes fail to adopt the T cell lineage
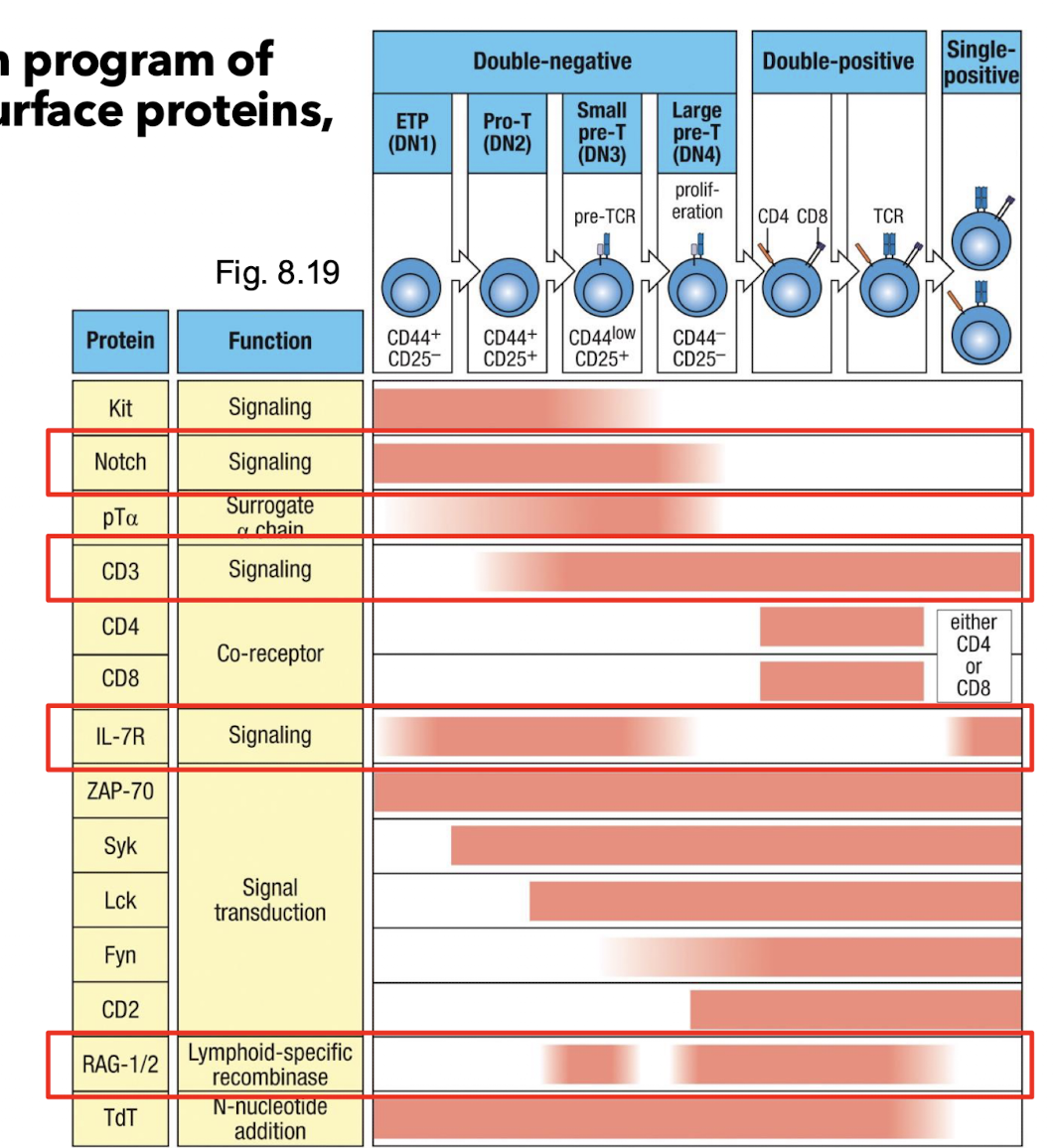
When is the CD3 expressed?
tells cell to single through your TCR
appears at DN → DP transition
part of the TCR complex, responsible for signal transduction once the TCR recognizes peptide MHC
indicates that the Pre TCR or TCR complex is forming and functional
When is the IL-7R expressed?
stay alive and divide
expressed mainly in early DN (DN1-DN3)
provide surveil and proliferation signals during early thymocyte growth
down regulated in DP since IL7 signalling is no longer needed once the TCR selection begins
When is the RAG1/2 expressed?
build your TCR
active during DN2-DN and again at DP
incomes enzymes that cut and joint the V(D)J segments during TCR gene rearrangement
turned off after successful rearrangement to prevent further DNA breaks
Why are Pre-TCRs important?
indicates successful (productive) TCR beta chain rearrangement
drives proliferation and maturation fo thymocytes
signals without a ligand (constitutive signalling)
stops further beta chain rearrangement. → maintains allelic exclusion
triggers TCR alpha chain rearrangement
prompts Transition to teh CD4+CD8+ DP stage
What is allelic exclusion?
Once a productive rearrangement at the β-chain locus has taken place (as tested by the formation of a functioning pre-T-cell receptor), events occur to prevent further recombination of the β chain. This recombination shutdown is referred to as allelic exclusion. This process is vital because it prevents the thymocyte from producing more than one functional β chain as it continues development. → why each only had one type of TCR
What is the beta chain checkpoint?
Pre-TCR = β-chain checkpoint that drives proliferation and triggers α-chain rearrangement.
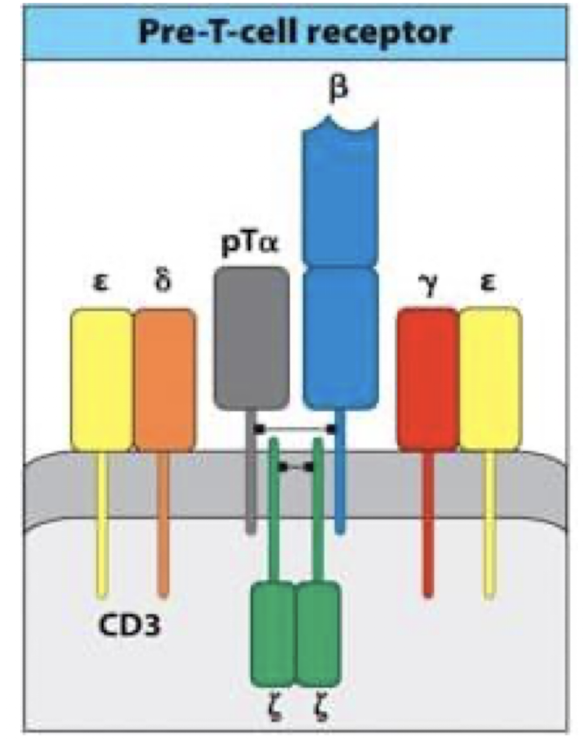
What happens after beta chain rearrangmeent>
DN3 cells progress to DN4
both CD4 and CD8 are expressed → now cells are DP
expression of CD4 and CD8 initiates the rearrangement of alpha chain locus in DP cells → alpha chain pair with the already functional beta chain formed a complete alpha beta TCR on cell surface
What marks the start of the selection process?
At DP stage, TCR and associated CD3 complex are expressed at low levels, just enough for the cell
to test whether the receptor can recognize self-MHC molecules presenting self-peptides.
This marks the start of the selection process
What is positive selection?
thymocytes that can moderate recognize self MHC and receive survival signals → ones we keep
What is negative selection?
while those that cannot or react too strongly will die → ones we get rid of
developmental stages within the cortex?
Stages of gene rearrangement in alpha beta T cells/
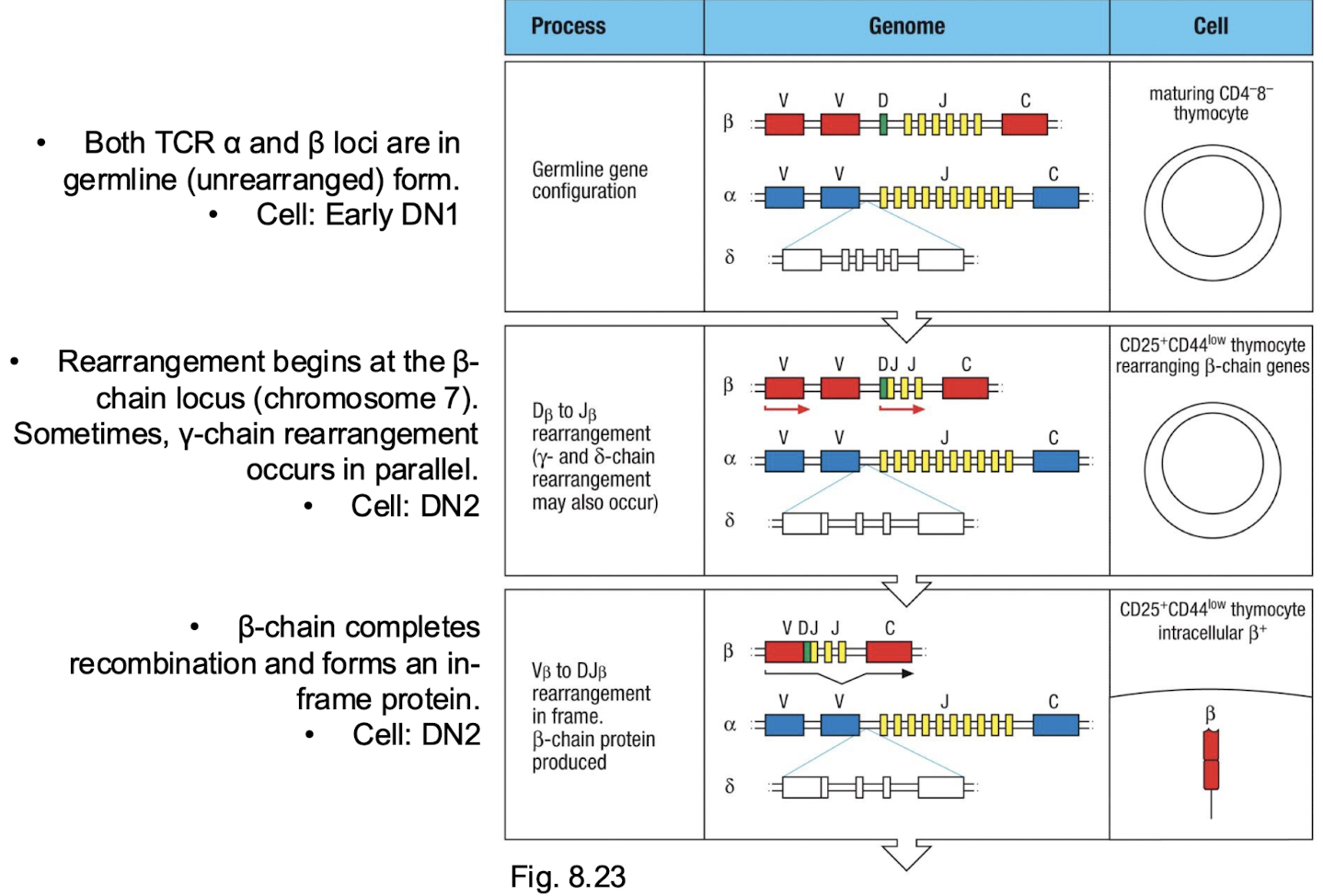
What are the 4 attempts to make a function beta chain?
TCR β locus contains two separate clusters of Dβ, Jβ, and Cβ segments (Dβ1–Jβ1– Cβ1 and Dβ2–Jβ2–Cβ2).
Each allele can attempt rearrangement in both clusters - giving four total chances (2 alleles × 2 clusters).
If one rearrangement fails (out- of-frame), the thymocyte can try again on the other cluster or allele before apoptosis. This redundancy increases the likelihood of producing a productive β-chain before the cell dies
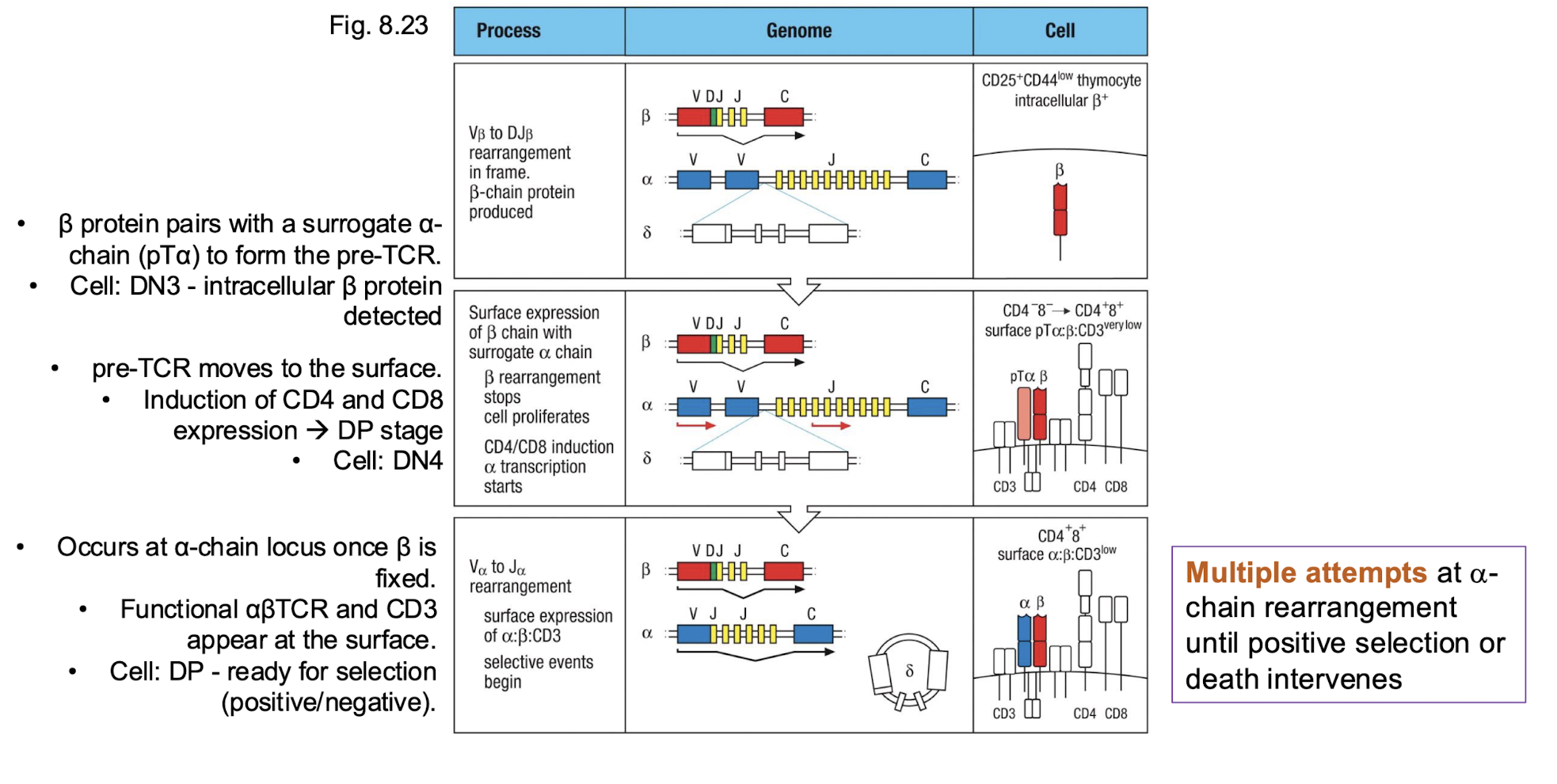
How many attempts do we get at alpha chain rearrangement?
Multiple attempts at alpha- chain rearrangement until positive selection or death intervenes →
Why do we have multiple attempts ate alpha chain rearrangement?
alpha chain locus have many V alpha and J alpha gene segments arranged linearly
allows for suquential recombination → if one is non function then the cell can try again using different V alpha and J alpha combination upstream or downstream
each new arrangement replaces the previous one on the same allele
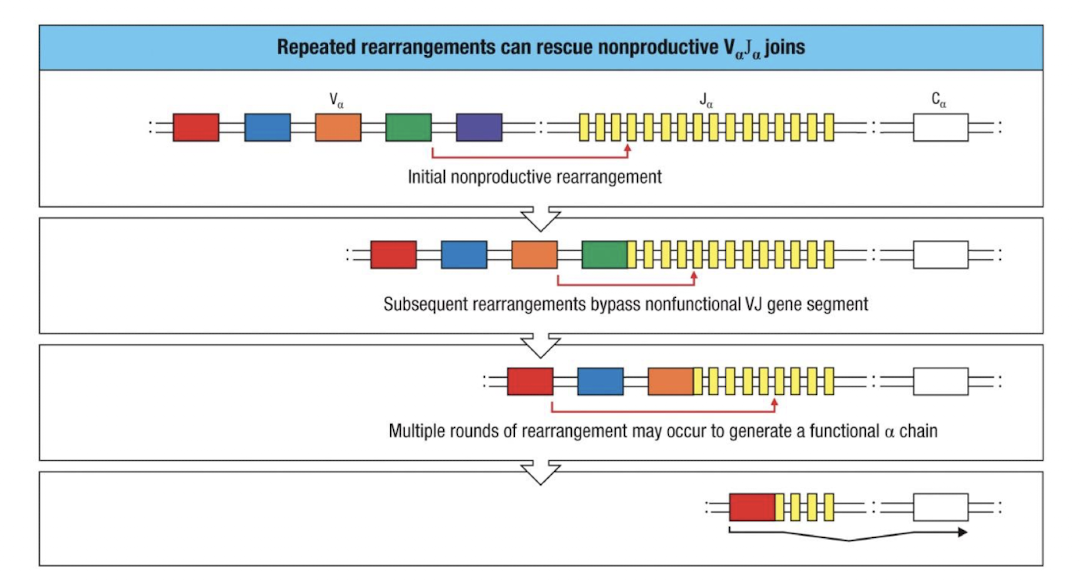
alpha chain rearrangement continues till…
The thymocyte successfully forms a functional αβ TCR that can recognize self-MHC (positive selection), or
It fails repeatedly and dies by apoptosis.
What are the 3 checkpoints
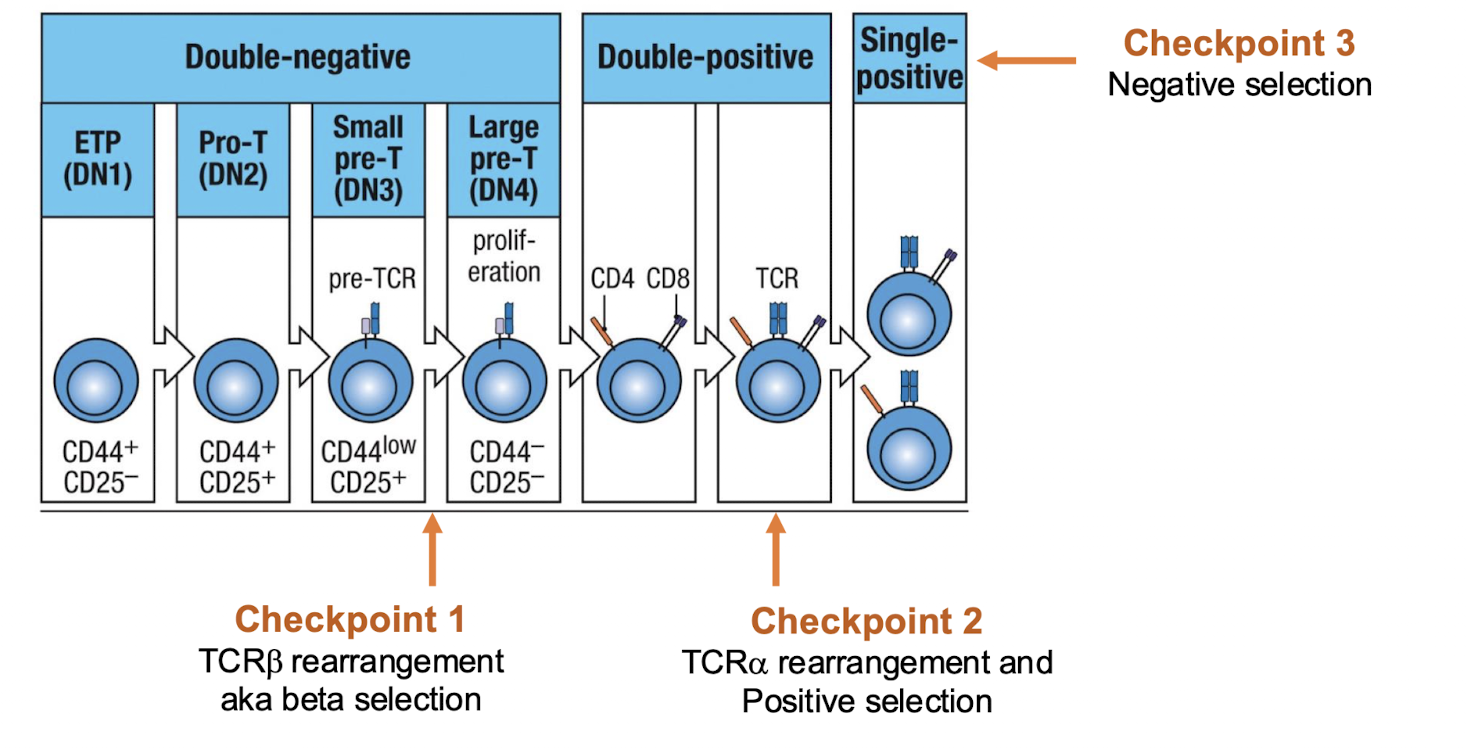
she is going to fix this diagram
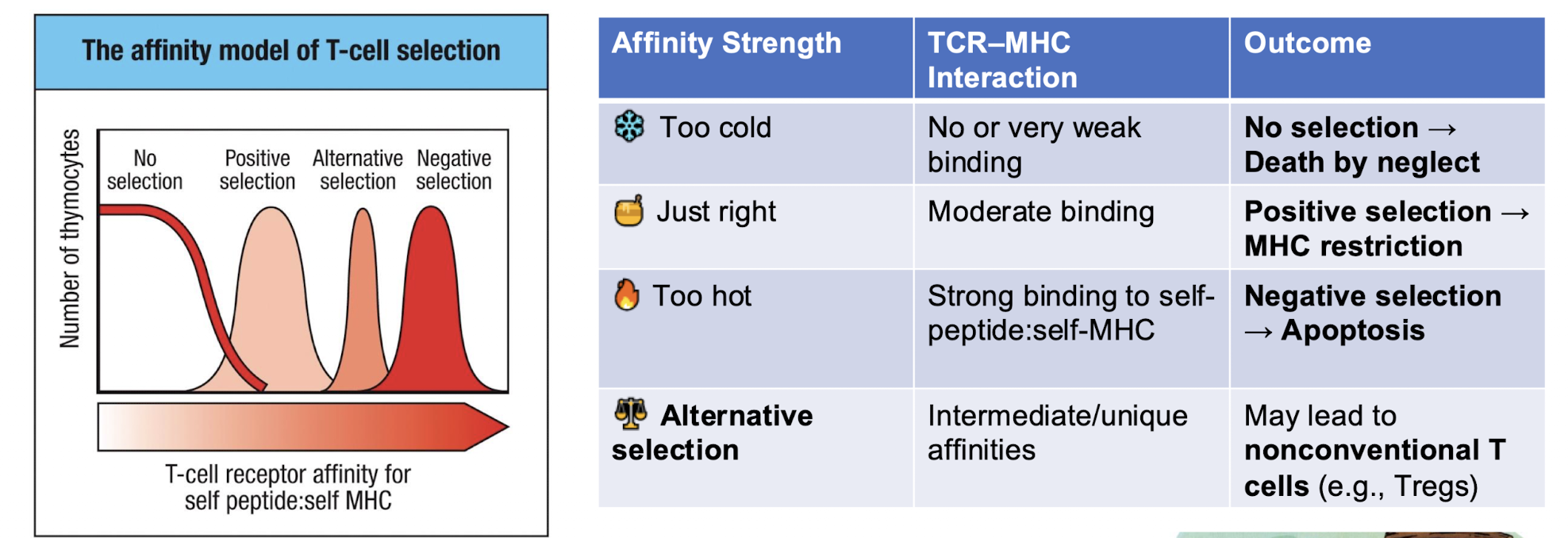
What is the goal of positive and negative selection
produce T cells that recognize foreign antigens presenting on self MHC molecules but ignore self
What is positive selection?
Occurs in thymic cortex
• Keeps only T cells that can bind to self-MHC (class I or II) → can it even bind an MHC because if it cant then we don’t want it
• Those that cannot bind die by neglect
• Results in MHC restriction
Cells that bind moderately survive and mature
• Cells that fail to bind die by neglect
• Cells that bind too tightly are flagged as potentially self-reactive
and later eliminated during NEGATIVE SELECTION in the cortex
• MHC recognition also determines lineage:
MHC class I → CD8⁺ cytotoxic T cells
MHC class II → CD4⁺ helper T cells
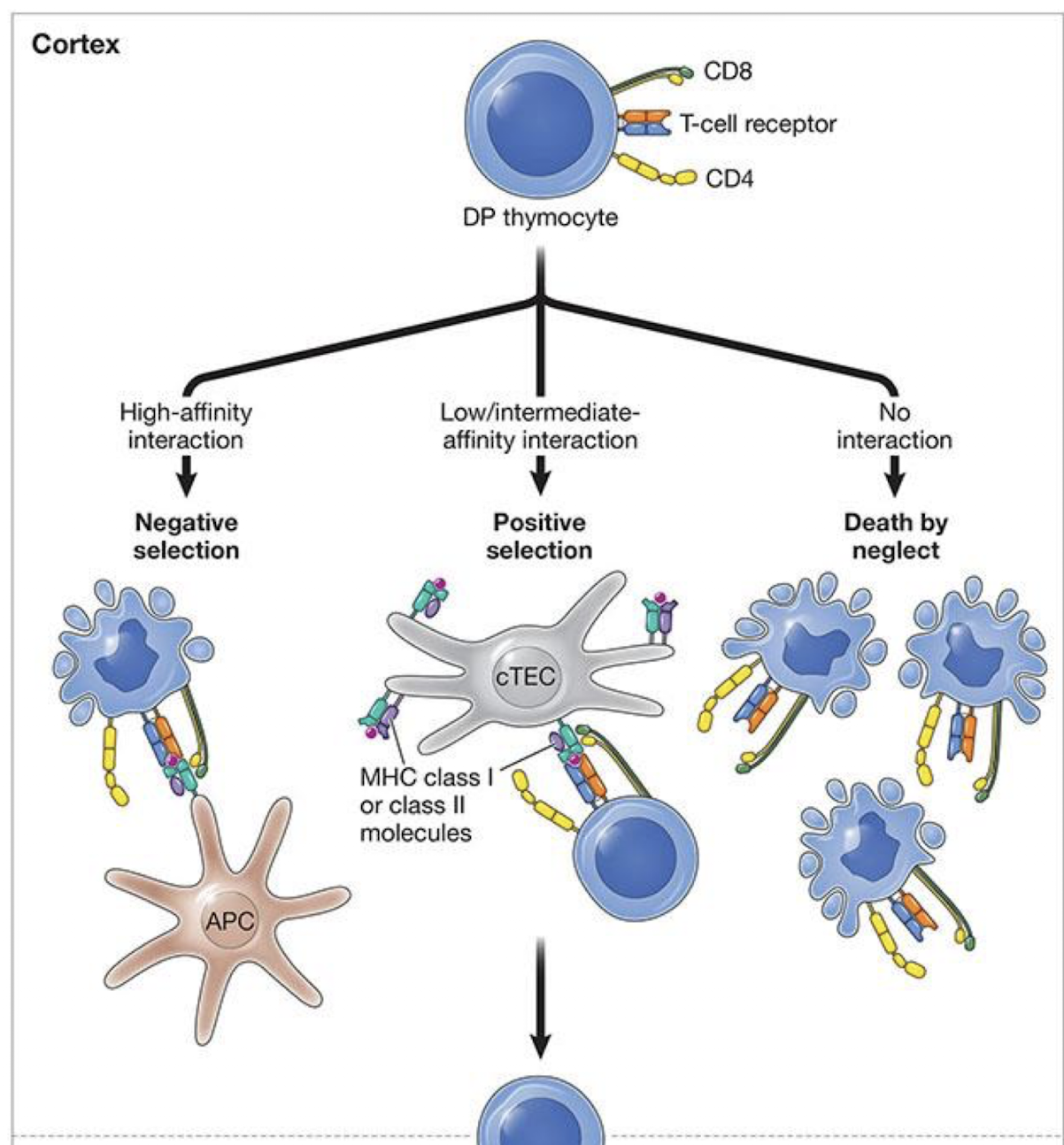
What is negative selection
Occurs both in cortex and medulla
• Removes T cells whose TCR bind self-peptide: self-MHC too strongly. →
• These cells die by apoptosis in thymus
• Results in self-tolerance
SP are screened by medullary
thymic epithelial cells (mTECs) and DCs
• Cells that bind self-peptide:self-MHC too strongly die by apoptosi
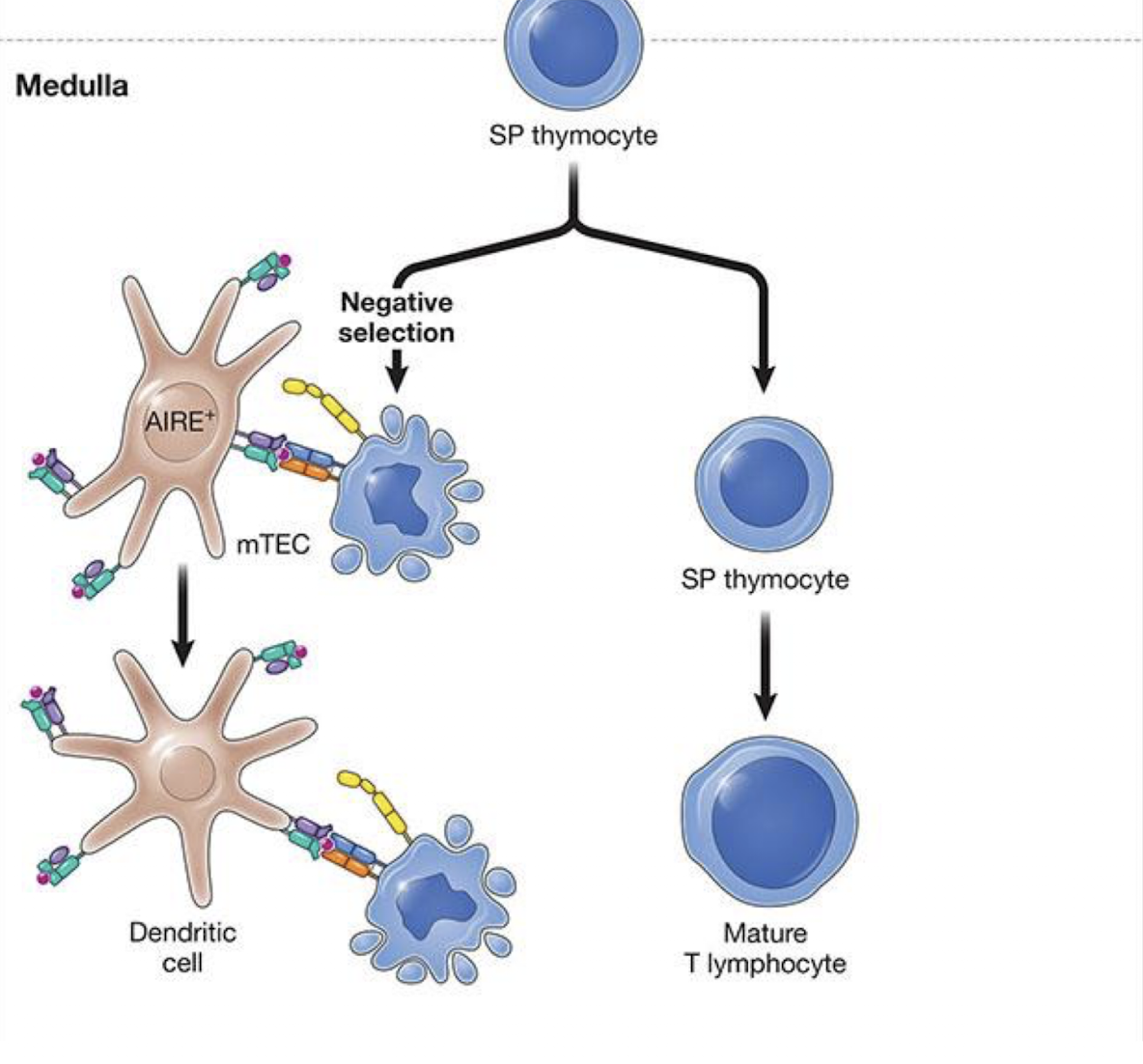
what is the affinity model of T cell selection
What is AIRE?
autoimmune regulator → enables thymus epithail cells to undergo promiscuous gene expression → enables presentation of tissue specific (tissues out side of the thymus), self antigens (or peripheral antigens) across the body (eg: insulin, myelin)
established central tolerance (occurring in primary lymphoid organs) and prevents autoimmunity
Key point: AIRE lets the thymus show T cells self-antigens from across the body - ensuring self-reactive cells are deleted – central tolerance
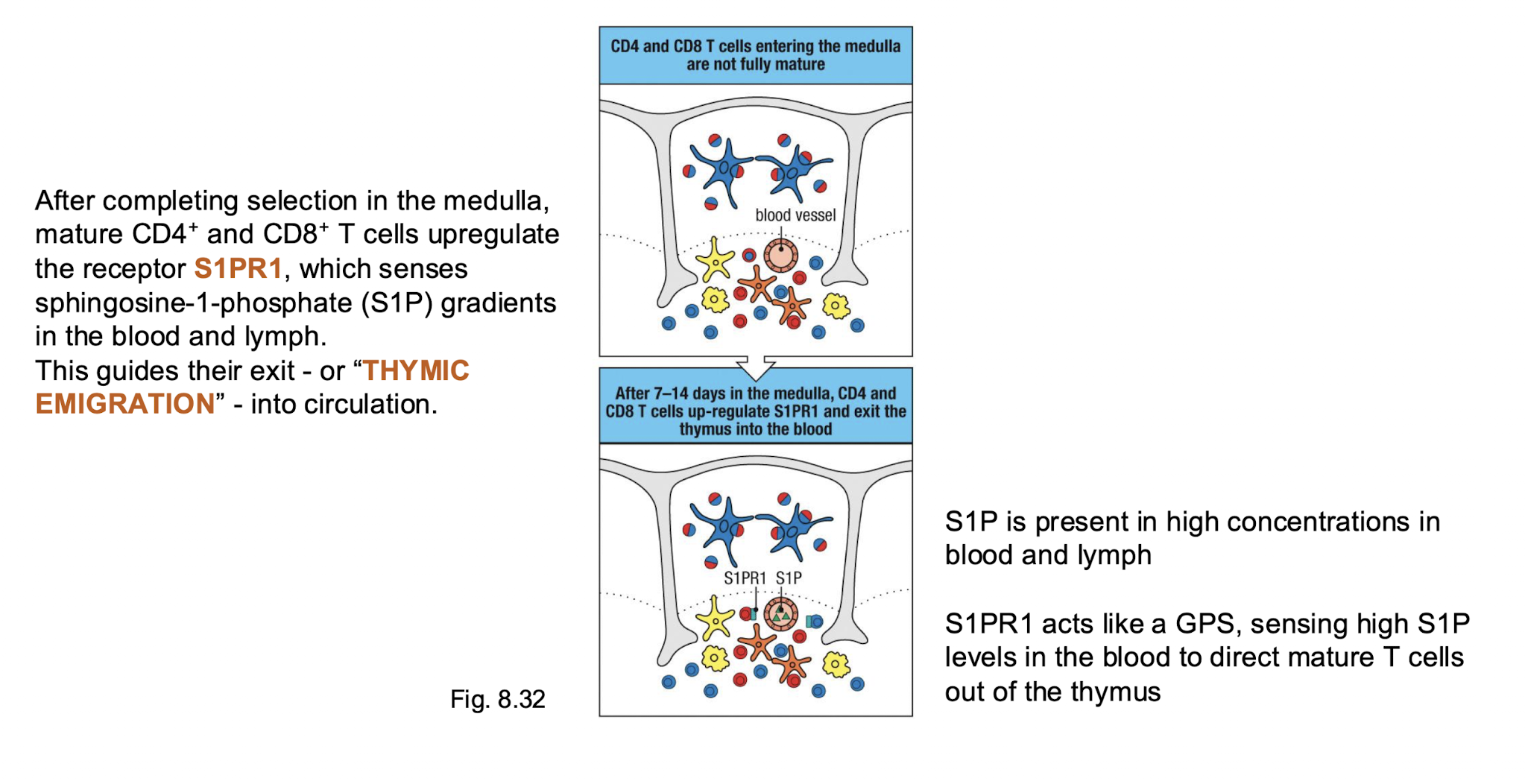
What induced thymus emigration