11 - Post-translational regulation
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
function of molecular chaperones
assist protein folding to lead to the correct (and most stable) form
how do Molecular chaperones work
• Chaperones repeatedly bind and release partially folded regions
• An exposed hydrophobic region can be bound by molecular chaperones to prevent aggregation until the protein has had a chance to fold correctly
Where should regions rich in hydrophobic amino acids mostly be found in a correctly folded protein?
towards the inside
Expression of molecular chaperones increases in response to heat. Why?
more heat = denature of proteins = need more help from molecular chaperones
Proteasomes
abundant sites for protein degradation and are found in the cytoplasm and nucleus
The protease activity is isolated inside a hollow cylinder
Why is protease activity isolated? Doesn’t that make it less efficient to access proteins?
specificity; you dont want to accidently cleave a properly functioning protein
“Unfoldase” enzymes
unfold target proteins and thread them into the cylinder
signals for degradation in the proteasome
• Polyubiquitin & misfolded regions are signals for degradation in the proteasome
• Proteins can be marked for destruction by covalent addition of a polyubiquitin chain
why it could be said that proteasome compete with molecular chaperones
If chaperones fail to fold a protein, it’s targeted to the proteasome for degradation.
Proteasome protects the cell from toxic aggregates.
both systems act on the same pool of misfolded proteins, but with different outcomes:
Chaperones: "Let me try to fix it."
Proteasome: "Too far gone—destroy it."
role of ubiquitin in targeting proteins for degradation
a small protein that can be covalently added to different sites on proteins
role of ubiquitin ligases in targeting proteins for degradation
combination of enzymes that covalently adds Ubiquitin
identify possible mechanisms through which degradation process could be regulated
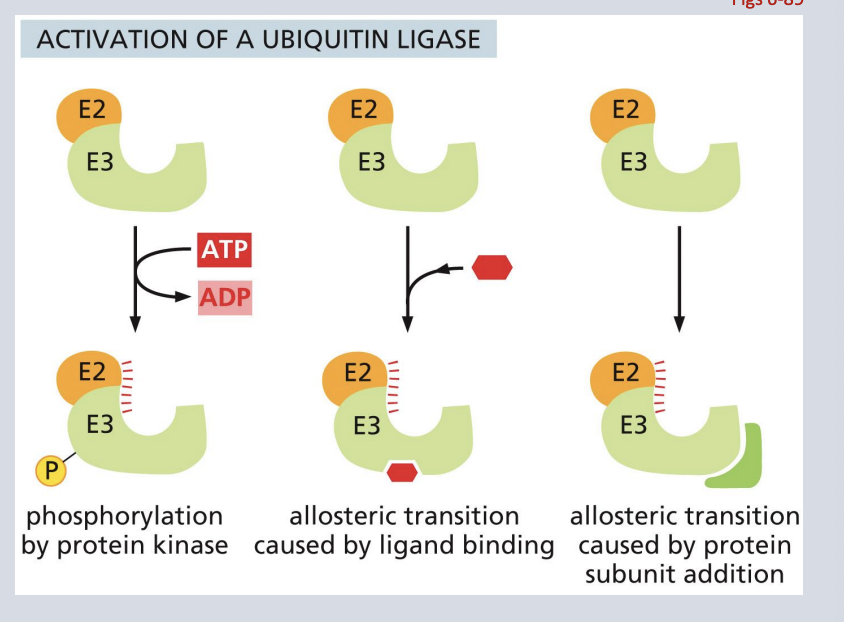
activation of a ubiquitin ligase
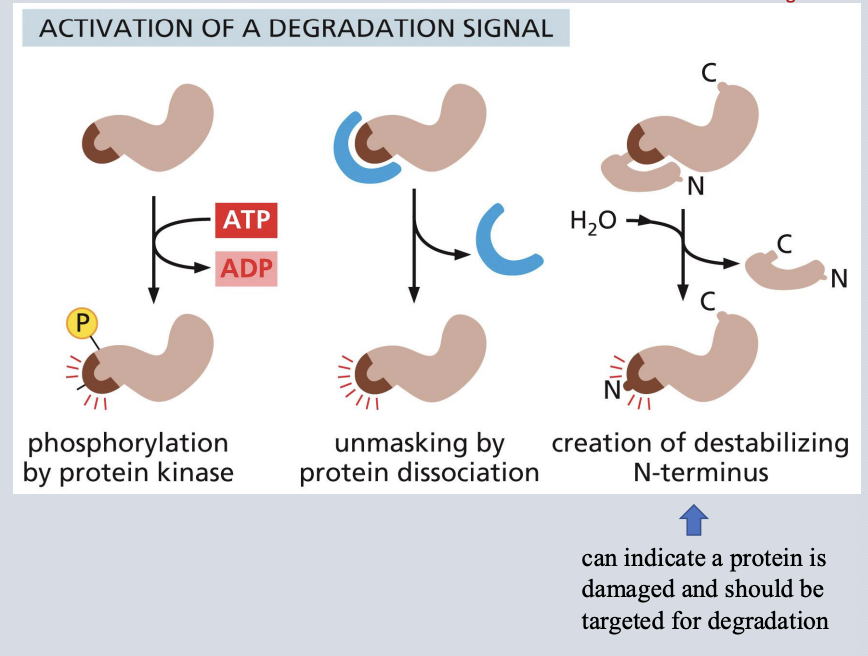
activation of a degradation signal
activation of a ubiquitin ligase
• Signals in the cell can activate a ubiquitin ligase complex (E2 and E3 shown here).
• Once activated, the ubiquitin ligase will bind to and ubiquitinate specific amino acid target sequences
• Proteins containing the ubiquitinated target amino acid sequence are then sent to the proteasome for degradation
activation of a degradation signal
• Signals in the cell result in creating a site that is recognized by a ubiquitin ligase
• Once the recognition site is created (ex. by phosphorylation or a change in conformation or complex dissociation) a ubiquitin ligase can target the protein for degradation
signal transduction
The signal is amplified and diversified (signaling branches to different pathways/ cell functions)
post-translational modification
chemical modification after translation that alters protein activity, stability, localization.
post-translational modification examples
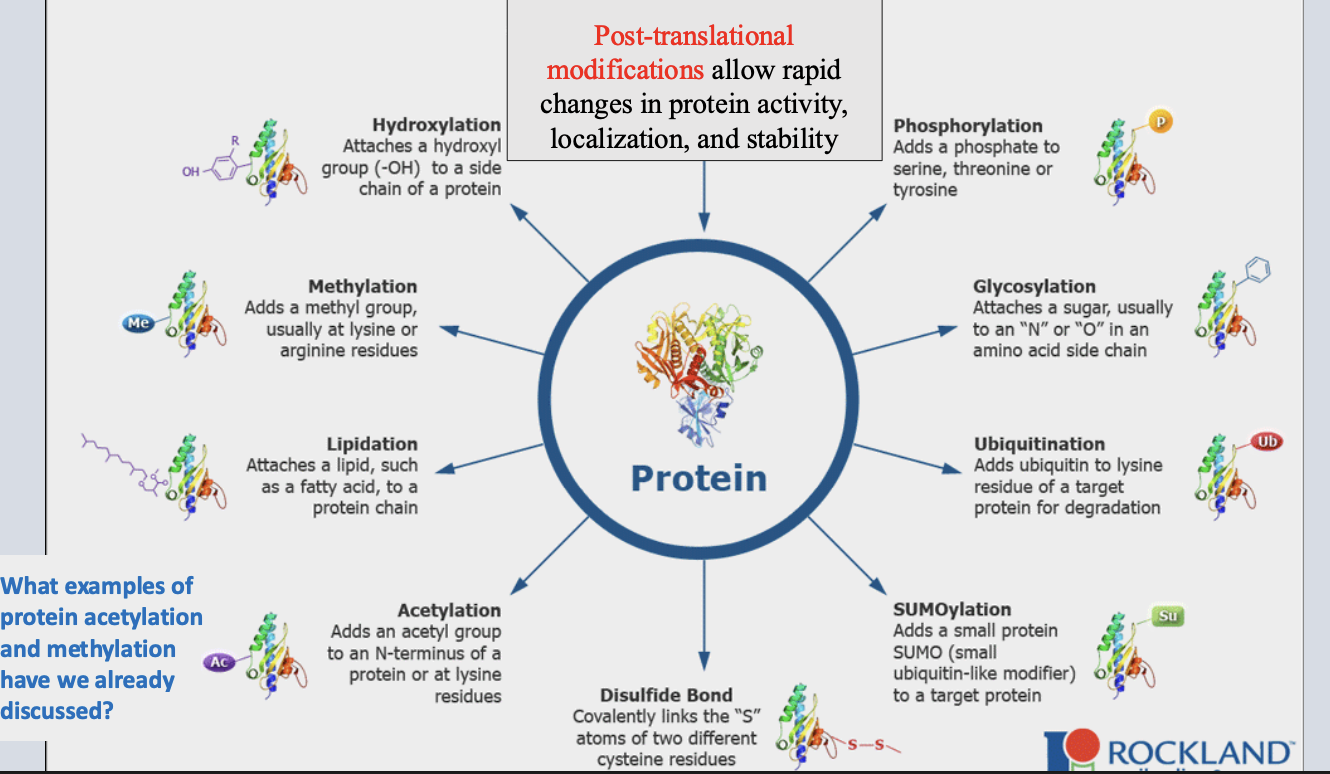
importance of Intercellular signaling
allows coordination between different cells/tissues
Types of cell -to -cell communication
paracrine, autocrine, juxtracrine, synaptic and endocrine
Endocrine signaling
ex. hormones; travel long distances in the blood
Paracrine signaling
involves secreted factors with a limited range (signal within a localized region)
Juxtacrine signaling
contact dependant
is mediated by contact NOT secreted, diffusible factors
autocrine
If the cell producing the signal is the same type as the cell receiving the signal, then the signaling can also be said to be autocrine
intracellular receptors
signaling receptors are located inside the cell
roles for Nuclear receptors
are usually transcription factors. Ligand binding results in a change in control of transcription (example: glucocorticoid receptor).
May initially be in cytosol but translocate to nucleus after ligand binding
describe roles for cell surface vs nuclear receptors
Cell surface receptors: bind extracellular ligands.
Nuclear receptors: bind ligands inside the cell (often transcription factors).
what effects cell signal
• The receptors expressed by a particular cell determine which signals it can receive
• The amount, duration, and combination of signals received together determine what effect a signal has on cell function
• The type of cell also influences the effect of receiving a particular signal
importance of signals
Some signals are required for the growth and survival of cells
Integrins
are transmembrane proteins that help cells adhere to the extracellular matrix (ECM)
Anoikis
a term for apoptosis that occurs in response to loss of adhesion (death due to lack of integrin signaling)
evidence that integrin can influence cell function/survival
Integrins connect cells to extracellular matrix (ECM).
Transmit survival and differentiation signals.
Loss of adhesion → anoikis (cell death).
Morphogens
are signaling molecules that diffuse from a localized source and form a concentration gradient across a developing tissue.
how can concentration of a signaling molecule influence cellular signaling responses
The amount of signal that reaches a cell is shaped by the synthesis/source, transport, and degradation of the paracrine factor.
Different concentrations of morphogen can activate different sets of genes in responding cells.
Why might morphogens be useful for building tissues?
to coordinate forming distinct tissues and building a multicellular orginism
are Signaling responses fast or slow
both
Many cellular signaling responses occur through regulation of gene expression (transcription) This takes time as the signal must be transmitted to the nucleus, then impact transcription and then impact translation
The fastest signaling responses involve direct modification of existing proteins to rapidly alter cellular processes
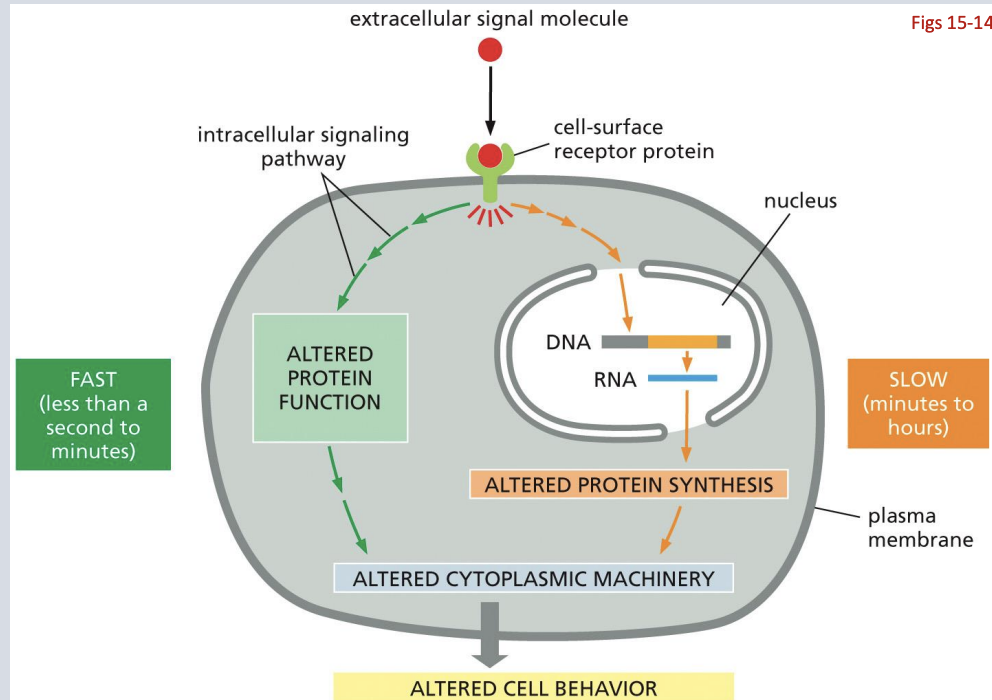
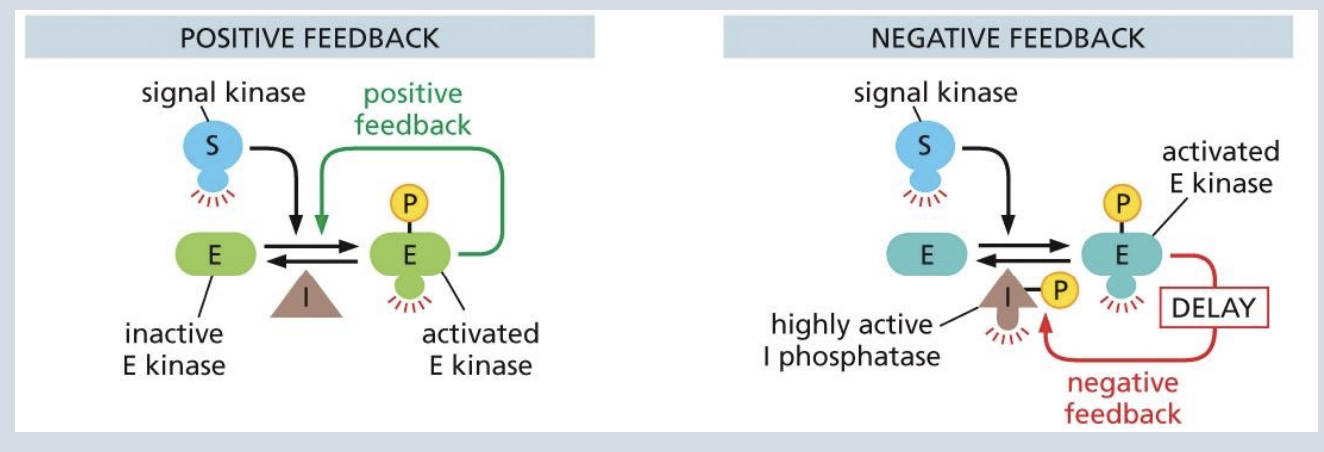
are Signaling pathways positive or negative feedback
Signaling pathways can include positive & negative feedback
“Molecular switches” in signal transduction: GTP binding
GTPases have different activity depending on whether they are bound to GTP or GDP
Generally, the GTP-bound form is active (”on”) in signal transduction pathways
Hydrolysis of GTP creates GDP and turns signaling “off
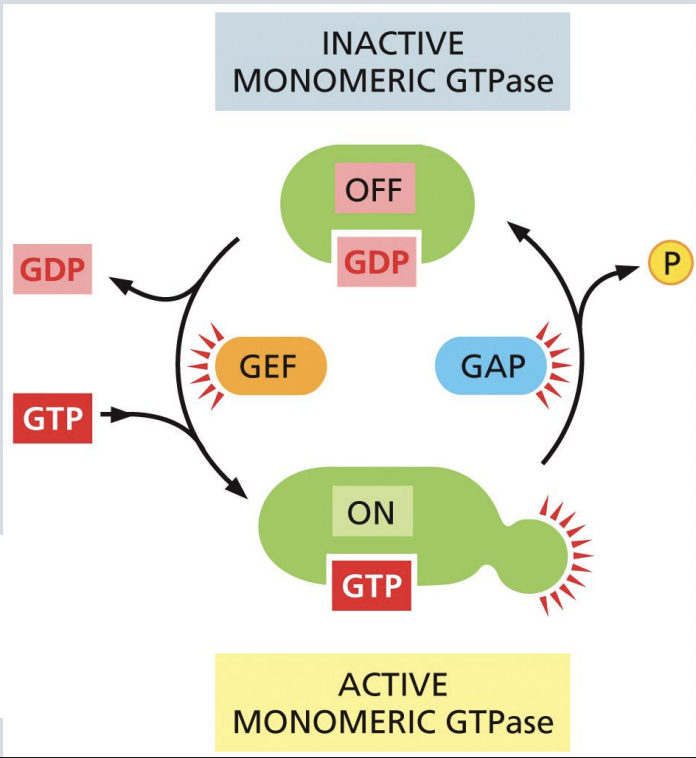
what promote GTP hydrolysis
GTP-ase activating proteins (GAPs)
what stimulate release of GDP so that GTP can bind.
Guanine nucleotide exchange factors (GEFs)
Mutations impacting GTPase signaling
are common in cancer (ex. in RAS GTPases) and create oncogenes with uncontrolled signaling
What types of changes could promote aberrant increased signaling of a GTPase?
deletion of gap
a mutation that impaired or slowed down the ability of KRas to hydrolyze GTP
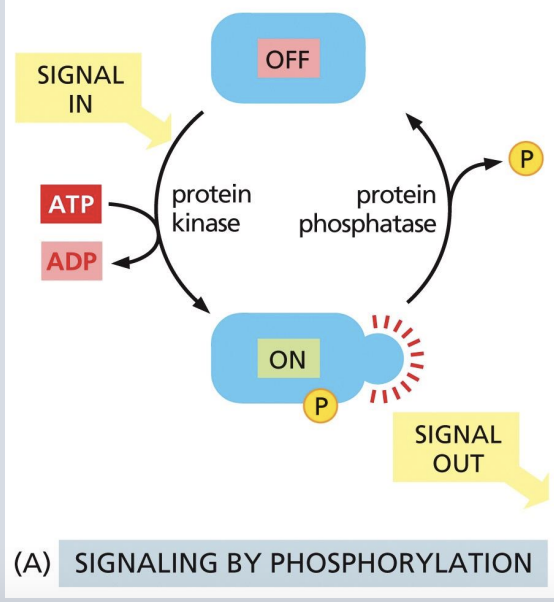
“Molecular switches” in signal transduction: Phosphorylation
Phosphorylation can change protein conformation, catalytic activity, and create/destroy binding sites for other interactions
Kinases add phosphate group from ATP to a particular amino acid.
Phosphatases remove the phosphate group.
Tyrosine kinases
phosphorylates tyrosine amino acids.
Serine/threonine kinases
phosphorylate serine and threonine amino acids
predict what types of mutations will produce oncogenes
Ras mutation that prevents GTP hydrolysis → always active.
Receptor tyrosine kinases mutated to be active without ligand.
Overactive kinases → excessive cell division.