Chemistry Need-to-Memorize
1/179
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
180 Terms
Giga
G, 10^9
mega
M, 10^6
kilo
k, 10^3
deci
d, 10^-1
centi
c, 10^-2
milli
m, 10^-3
micro
µ, 10^-6
nano
n, 10^-9
pico
p, 10^-12
femto
f, 10^-15
Farenheit Dimensional Analysis Equation
T (in ºF) = (9/5) (T(in ºC) + 32)
Celsius Dimensional Analysis Equation
T(in ºC)= (5/9) [T(in ºF) - 32]
kelvin Dimensional Analysis Equation
T(in K)= T(in ºC) + 273.15
1 inch is how many cms?
2.54cm
1kg is how many lb?
2.205 lb
1mL is how many cm?
1cm³
1ft is how many inches?
12 in
1yd is how many ft?
3 ft
What are the 5 sig fig rules
All nonzero digits are significant
Zeroes between other sigfigs are significant
Leading zeroes, which are only used to show the position of the decimal point, are not significant
Final zeroes in a= a number that has a decimal point are significant
Final zeroes in a number with no decimal points are not considered significant
SI Unit of charge
Coulomb (C)
Proton abbreviation
p, 1H+, H+ or, p+
Neutron abbreviation
n
Electron abbreviation
e-
Proton Mass (amu)
1.00727
Neutron Mass (amu)
1.00866
Neutron Charge (C)
0
Proton Charge (relative)
+1
Neutron Charge (relative)
0
Electron Charge (relative)
-1
Protons are located in the. . .
nucleus
Neutrons are located in the. . .
nucleus
Electrons are located in the. . .
outside the nucleus in a cloud “electron cloud”
Atomic Number
Z, the number of protons in an atom
Mass Number
A, The total number of protons and neutrons in an atom
Isotopes
atoms of the same element that have different mass numbers
How do I format writing Isotopes?
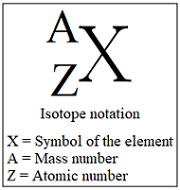
Cations
Positively charged ions, #p >#e-, positively charged
Anions
Negatively charged ions, #p < #e-, negatively charged
Atom
the smallest identifiable unit of an element, #p = #e-, neutral
Atomic mass units (amu) are also known as . . .
Daltons (Da)
How much is 1 amu?
1 amu = 1/12th of a Carbon-12 atom, 1amu= 1.660539 × 1027 kg
Atomic weight
The weighted average of the isotopic masses of the element’s naturally occurring isotopes
How do you find the weighted average?
(Isotopic Abundance * Isotopic Mass) + ((Isotopic Abundance * Isotopic Mass)
Silver
Ag
Aluminium
Al
Argon
Ar
Arsenic
As
Gold
Au
Boron
B
Barium
Ba
Beryllium
Be
Bromine
Br
Carbon
C
Calcium
Ca
Chlorine
Cl
Cobalt
Co
Chromium
Cr
Cesium
Cs
Copper
Cu
Fluorine
F
Iron
Fe
Gallium
Ga
Germanium
Ge
Hydrogen
H
Helium
He
Mercury
Hg
Iodine
I
Potassium
K
Krypton
Kr
Lithium
Li
Magnesium
Mg
Manganese
Mn
Molybdenum
Mo
Nitrogen
N
Sodium
Na
Neon
Ne
Nickel
Ni
Oxygen
O
Phosphorous
P
Lead
Pb
Platnium
Pt
Rubidium
Rb
Radon
Rn
Sulfur
S
Antimony
Sb
Scandium
Sc
Selenium
Se
Silicon
Si
Tin
Sn
Strontium
Sr
Tellurium
Te
Titanium
Ti
Uranium
U
Vanadium
V
Tungsten
W
Xenon
Xe
Zinc
Zn
H2O
Dihydrogen monoxide, Molecular Compound