2024-25 Atomic Structure 낱말 카드 | Quizlet
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Atom
a unit of matter, the smallest unit of an element, having all the characteristics of that element and consisting of a dense, central, positively charged nucleus surrounded by a system of electrons.
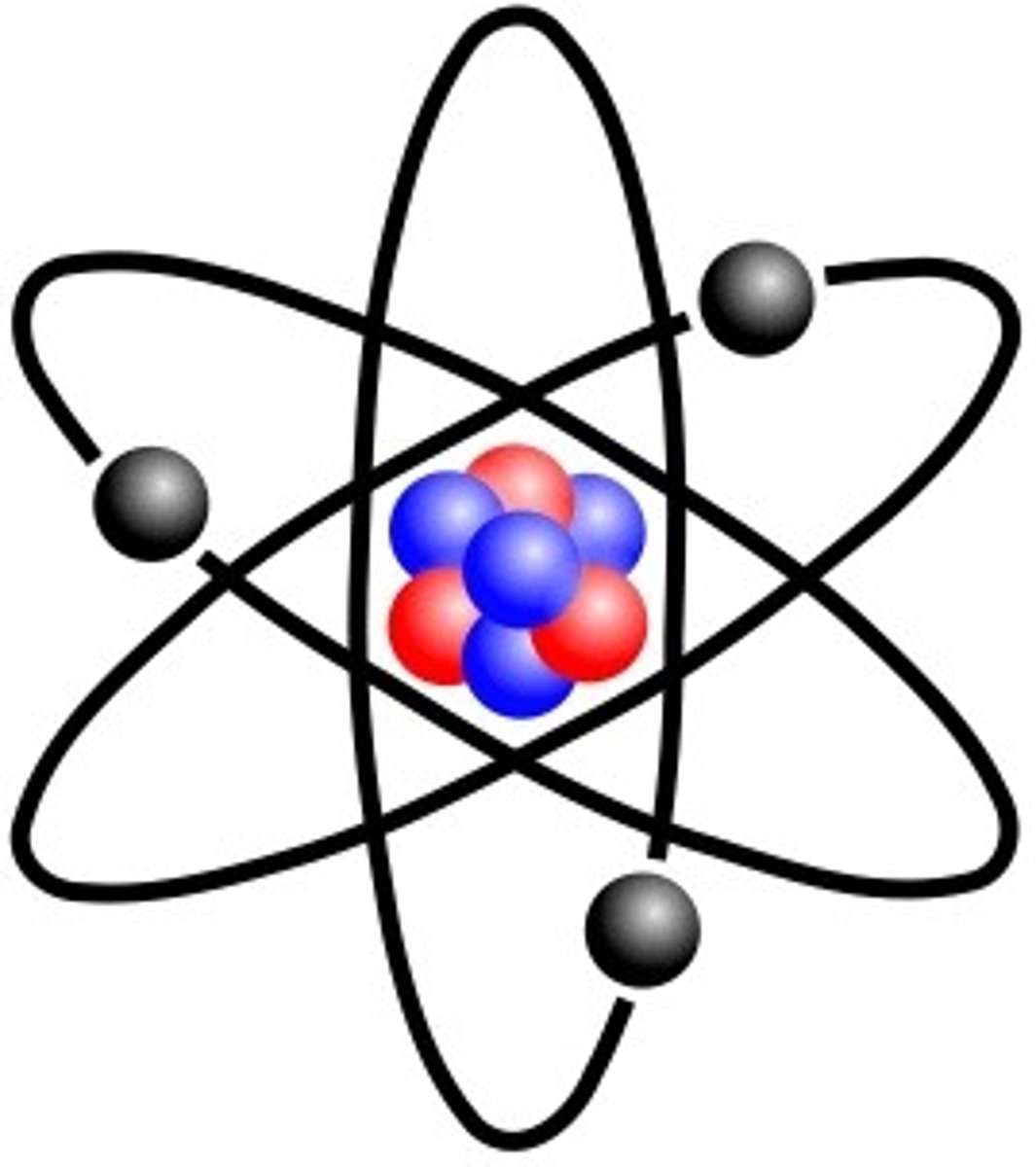
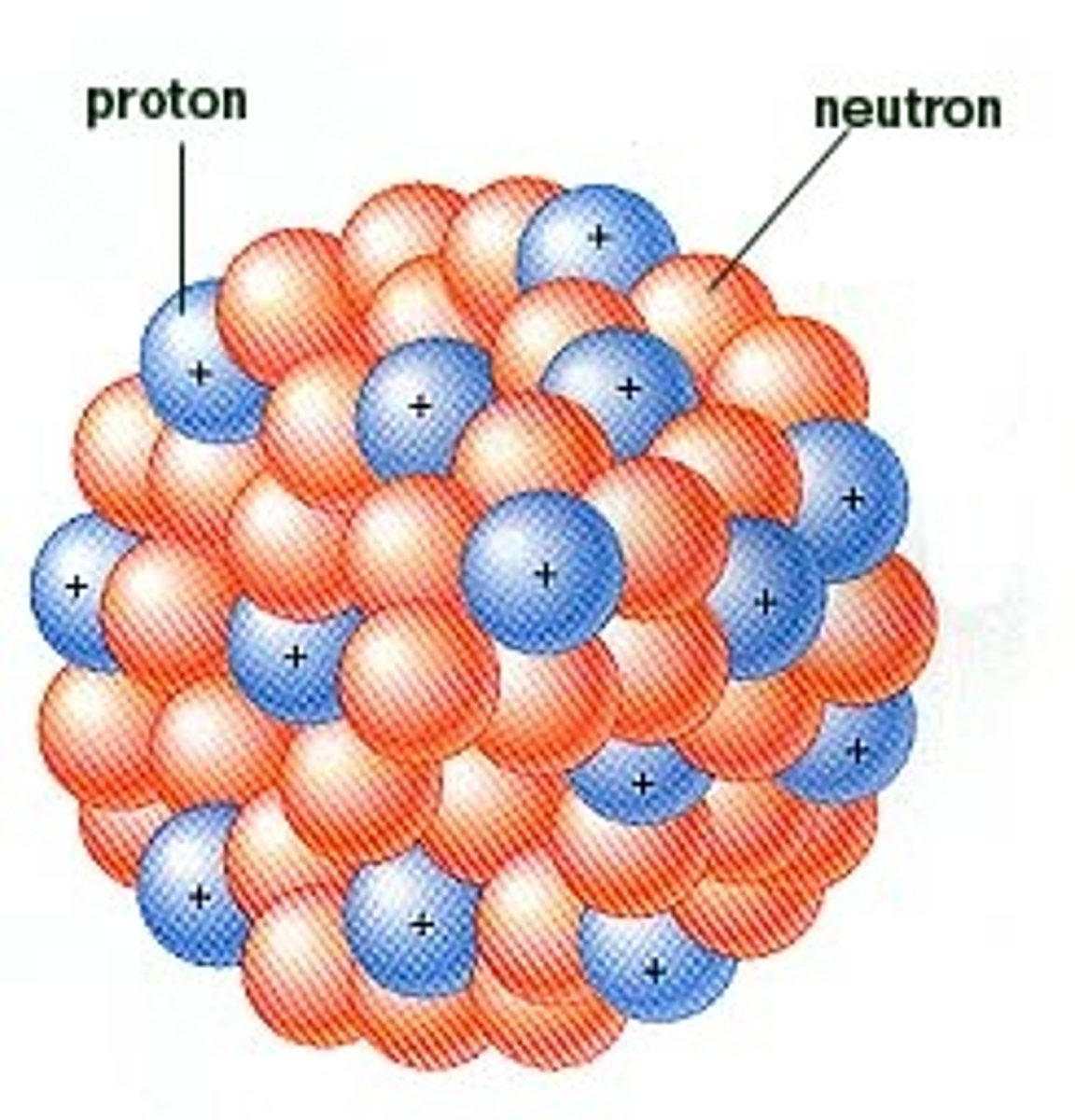
Proton
a subatomic particle with a positive charge located in the nucleus of an atom and has a mass of 1 amu (atomic mass units).
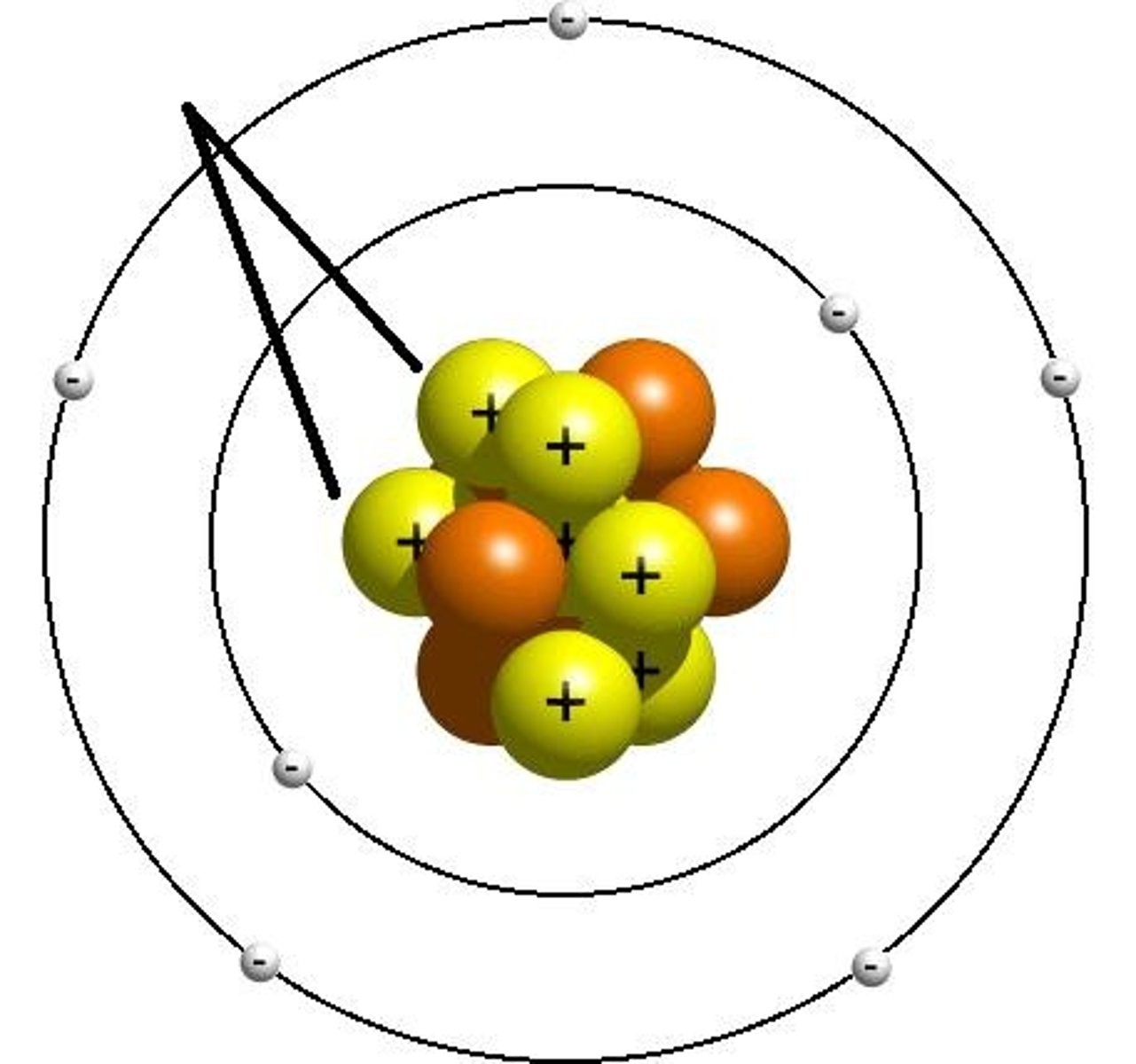
Neutron
a subatomic particle with a neutral charge located in the nucleus of an atom and has a mass of 1 amu (atomic mass units).
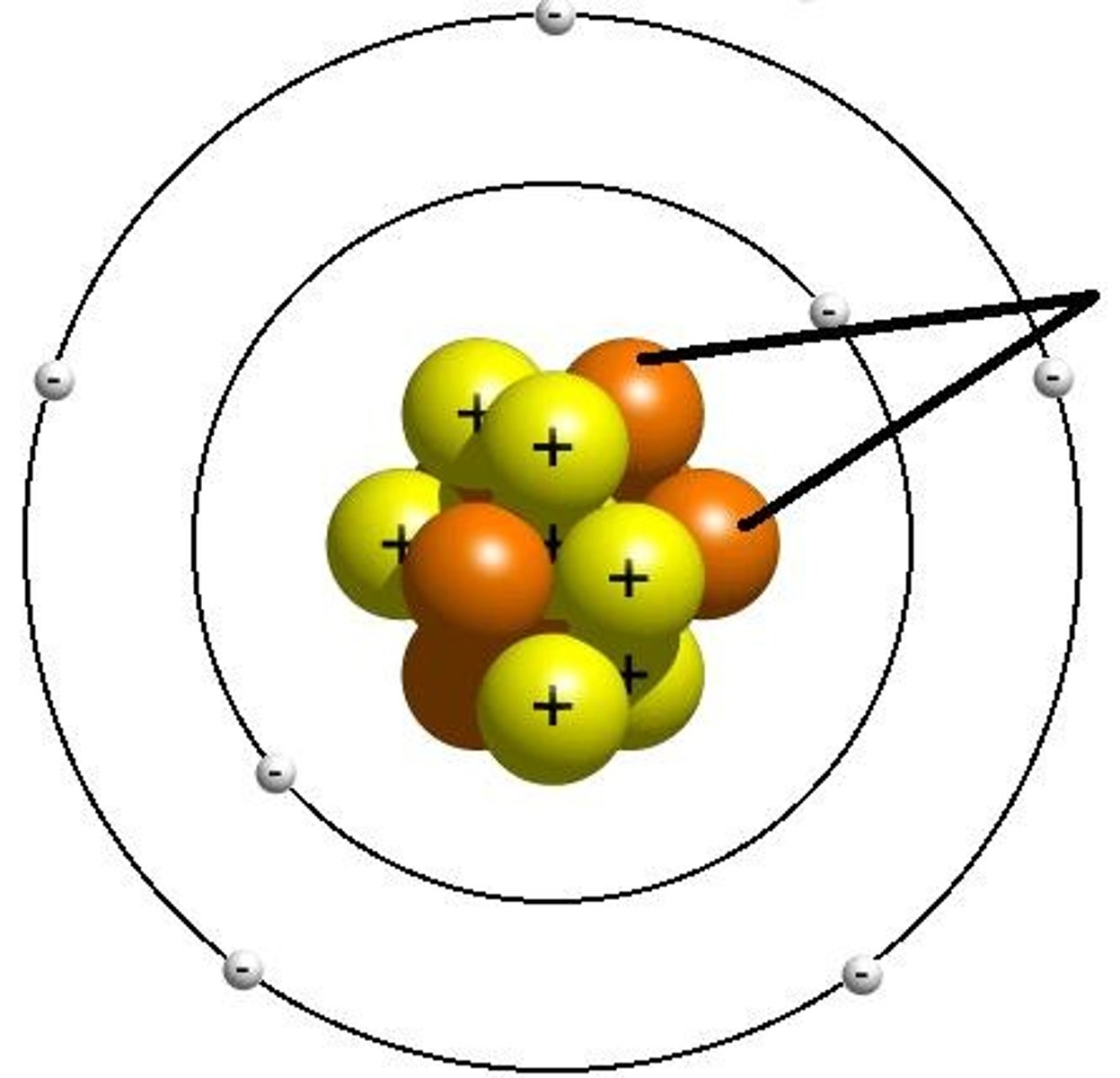
Electron
A subatomic particle that has a negative charge
Atomic mass unit (amu)
a unit of mass equal to one-twelfth the mass of a carbon-12 atom
Nucleus
the central part of the atom housing the protons and neutrons.
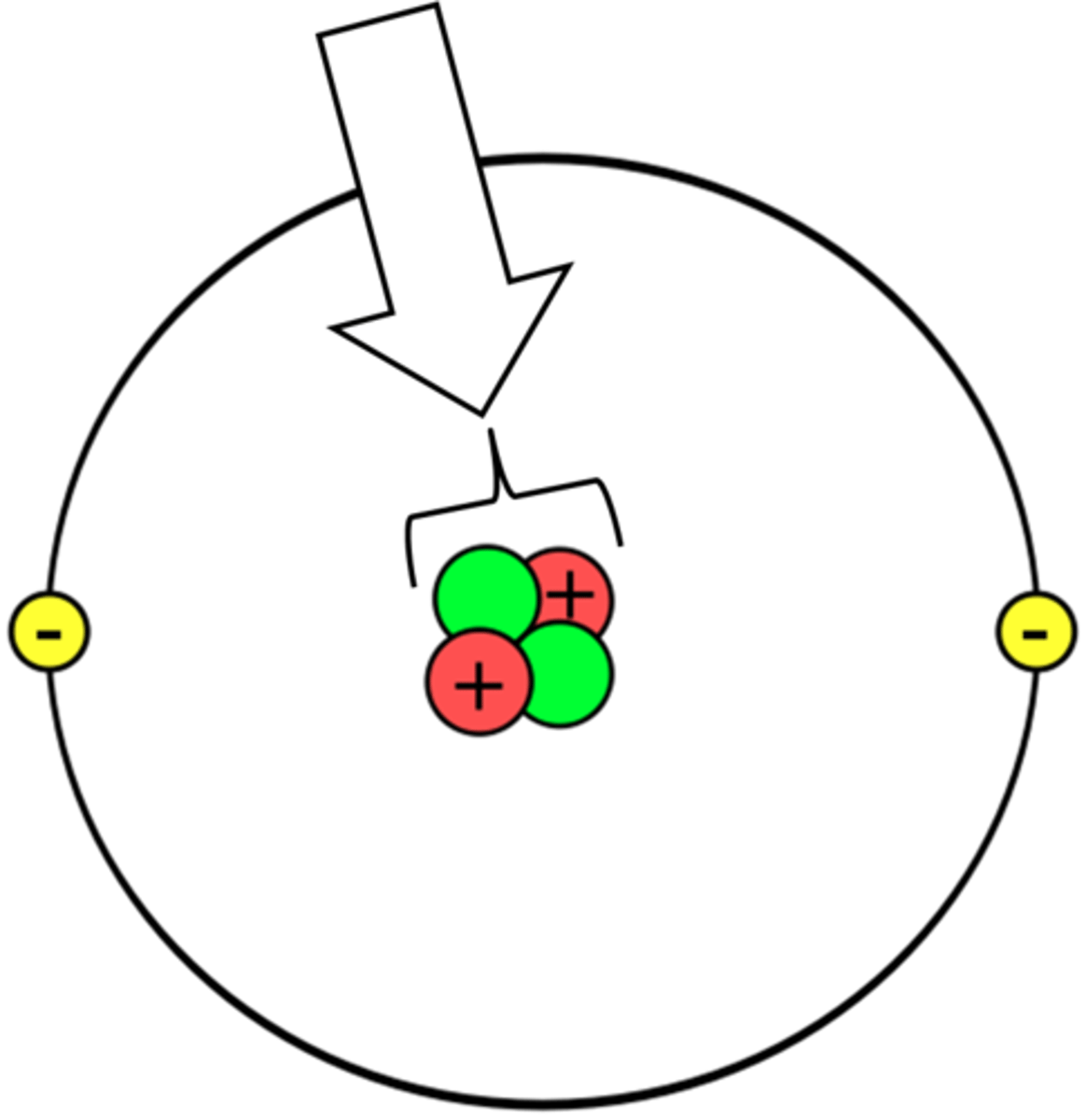
Atomic number
the number of protons in an atomic nucleus.

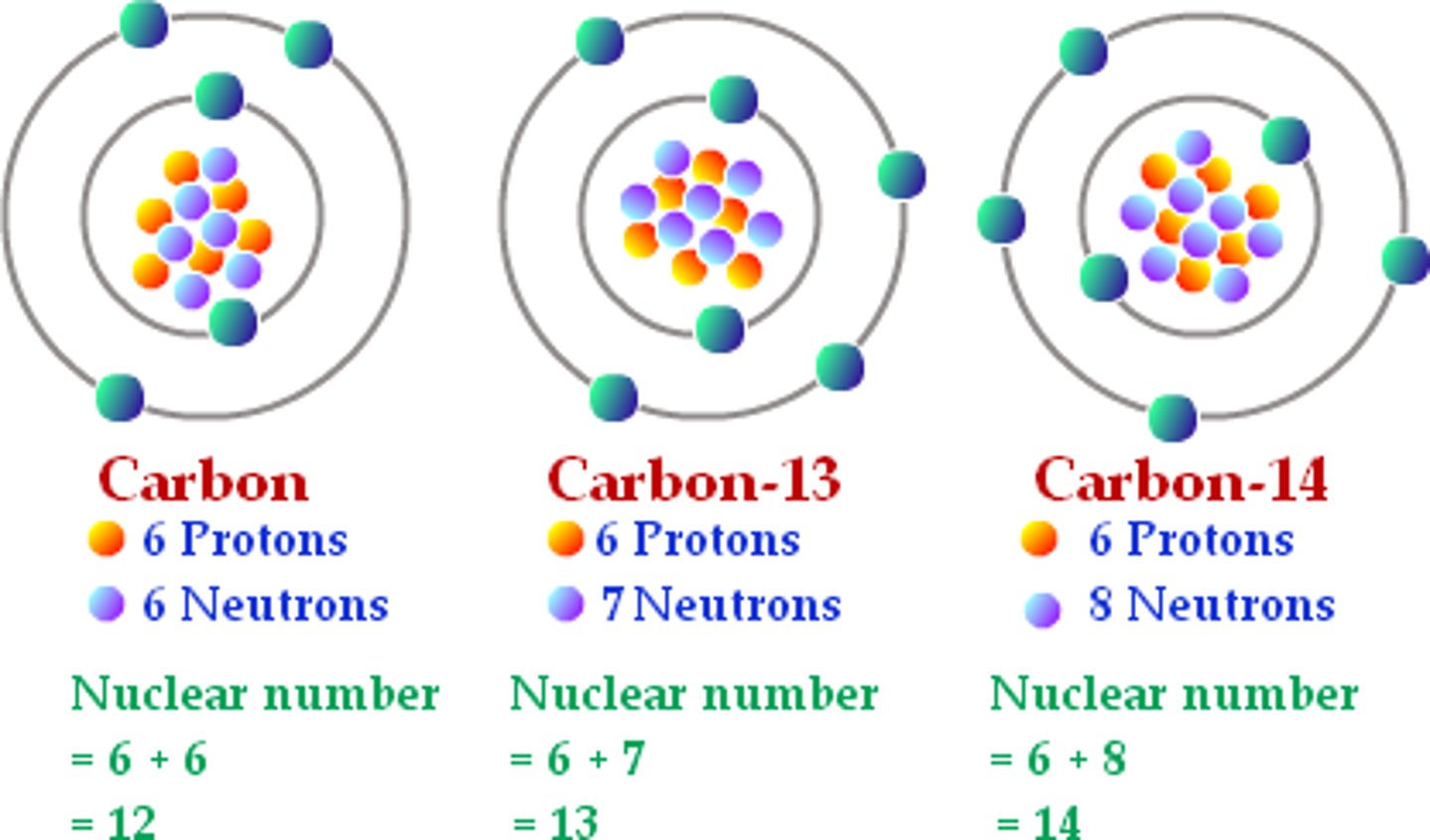
Isotope
Atoms of the same element that have different numbers of neutrons and a different Mass Number
Subatomic Particles
Protons, neutrons and electrons
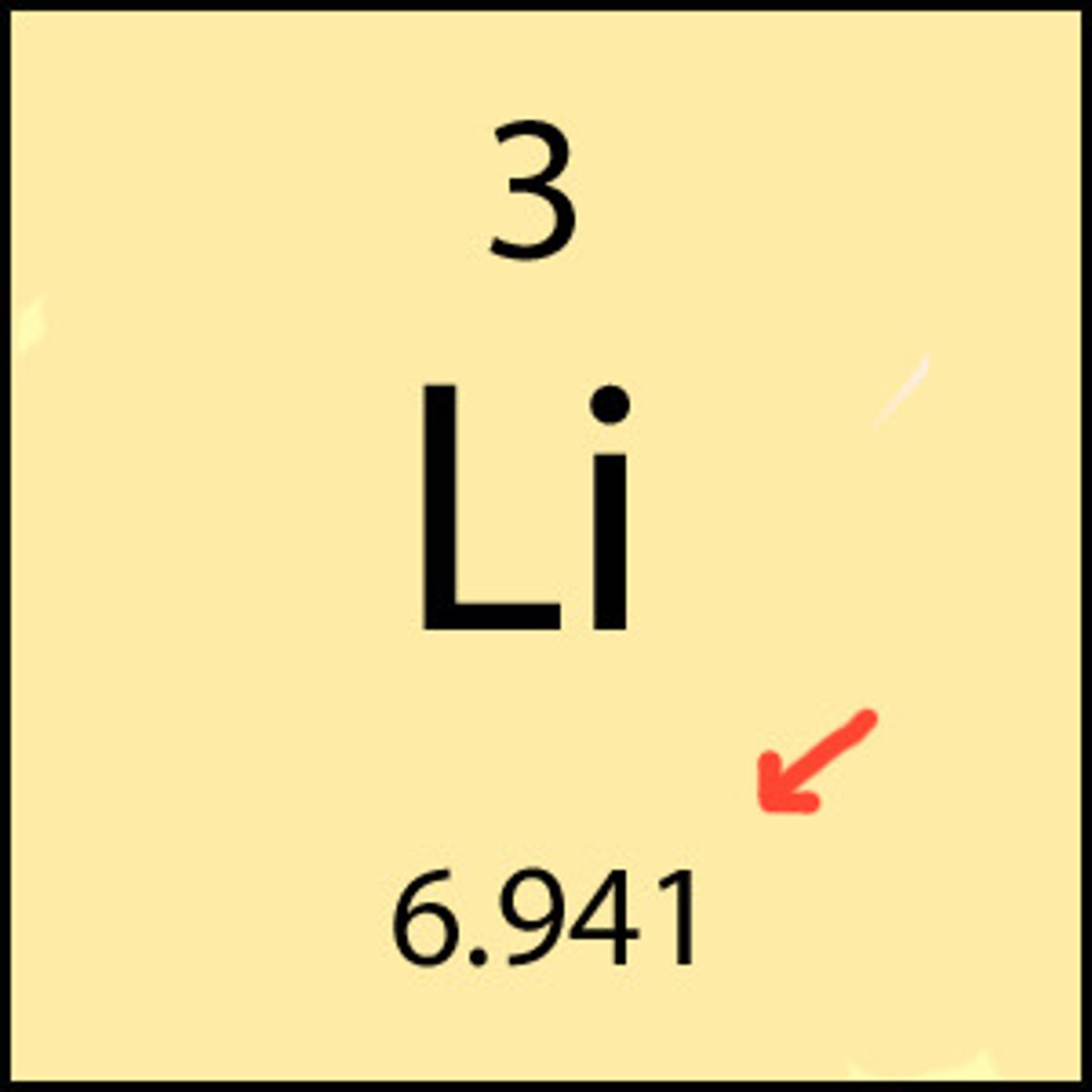
average atomic Mass
the average mass of all the different isotopes that make up the element. The average is calculated using the relative abundance of isotopes in a naturally occurring element.
Mass Number
the sum of the number of neutrons and protons in an atomic nucleus
Nuclear Symbol
The superscript indicates the mass number and the subscript indicates the atomic number
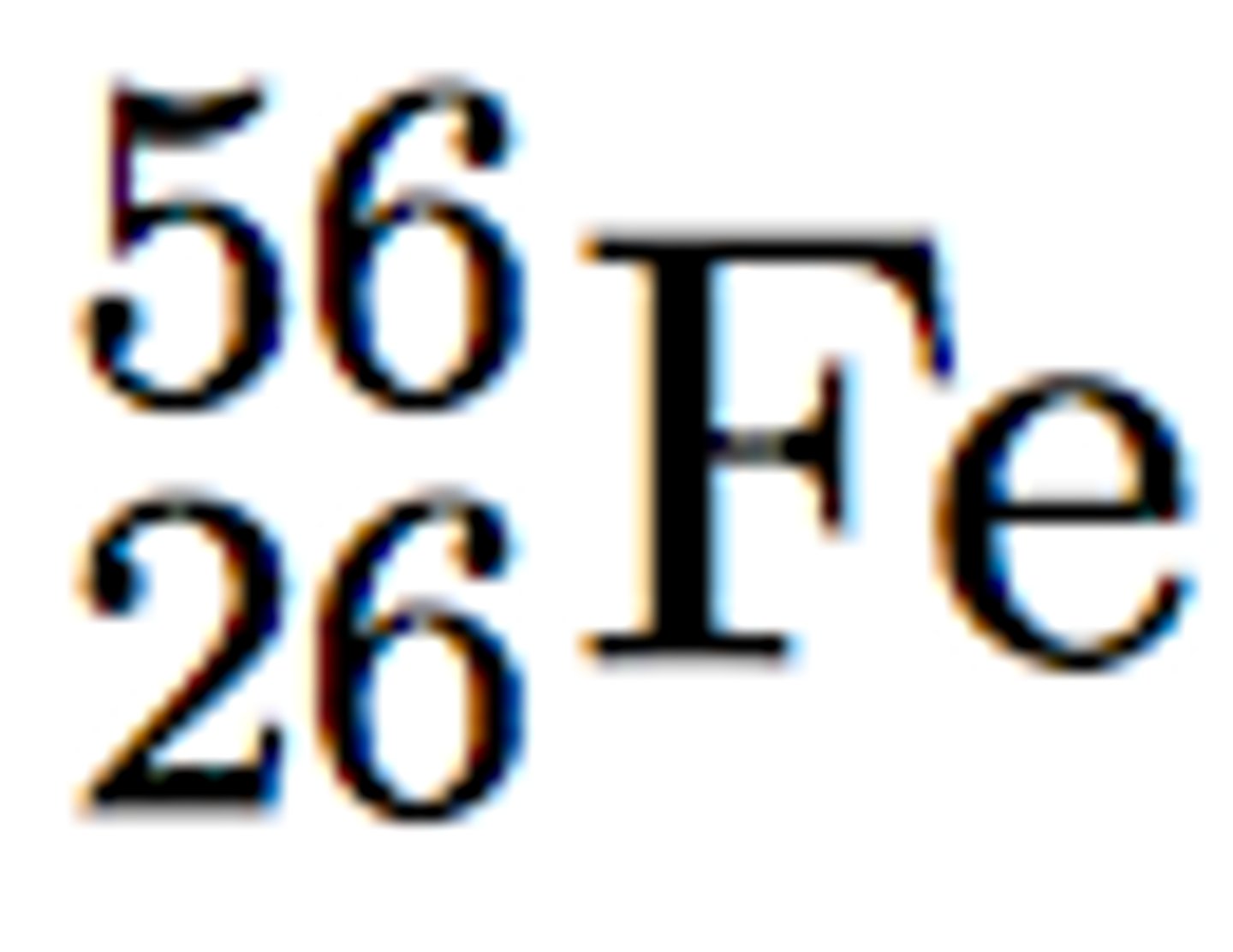
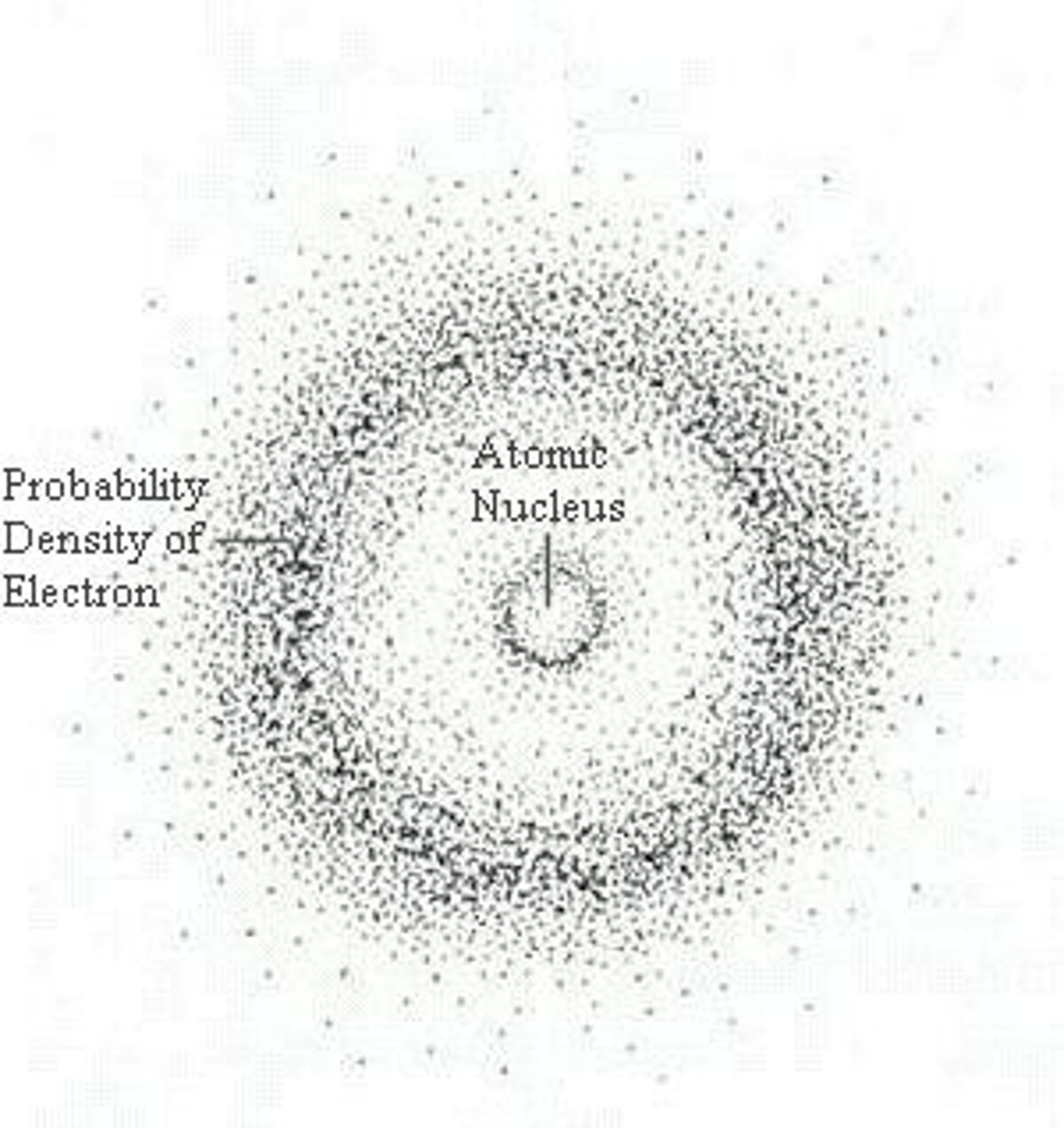
electron cloud
a region around the nucleus of an atom where electrons are likely to be found
hyphen notation
the mass number is written with a hyphen after the name of the element
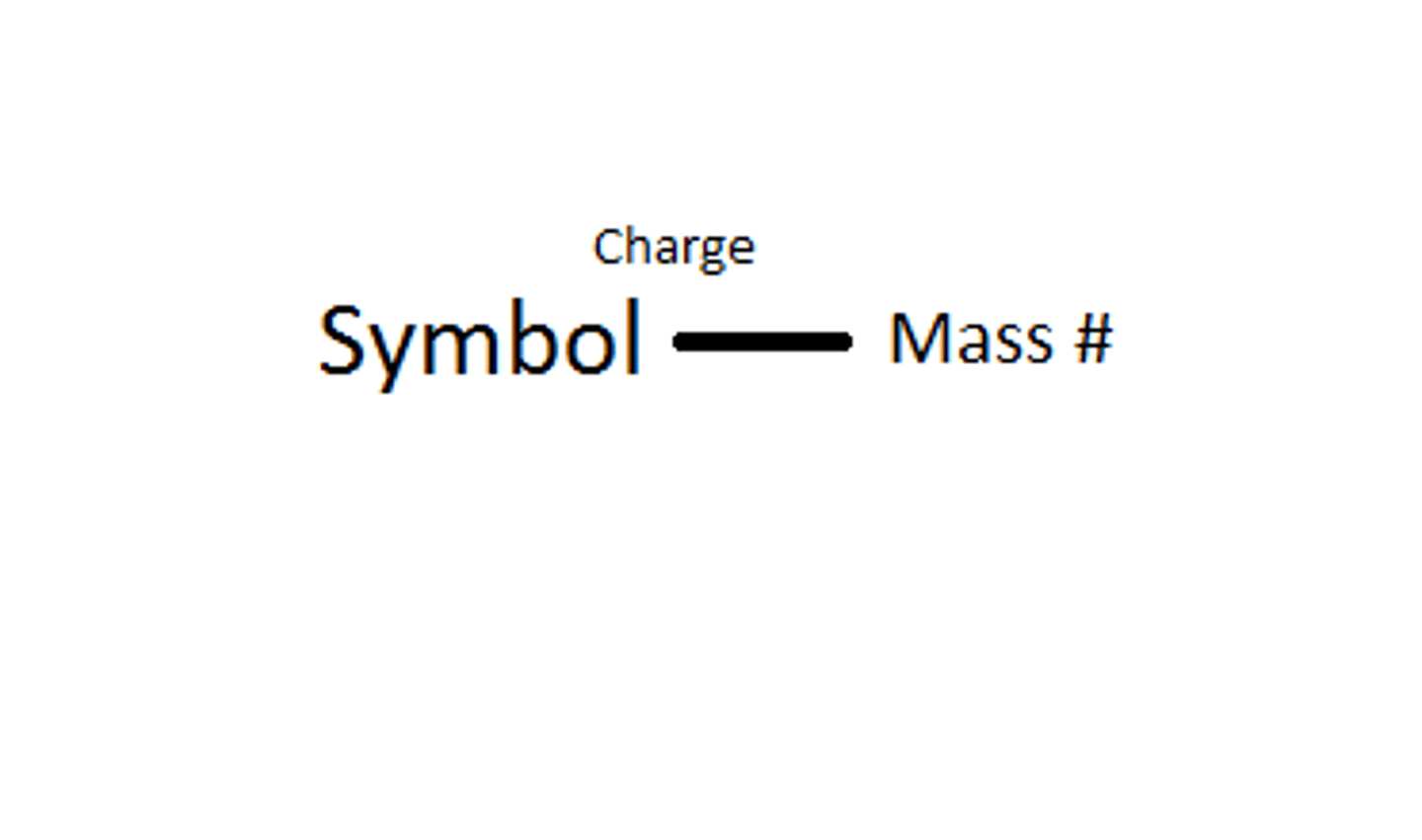
nucleon
Any subatomic particle found in the atomic nucleus. Another name for either a proton or a neutron.
subscript
A text format where text is written slightly below the surrounding text
superscript
A text format where text is raised and smaller than surrounding text.
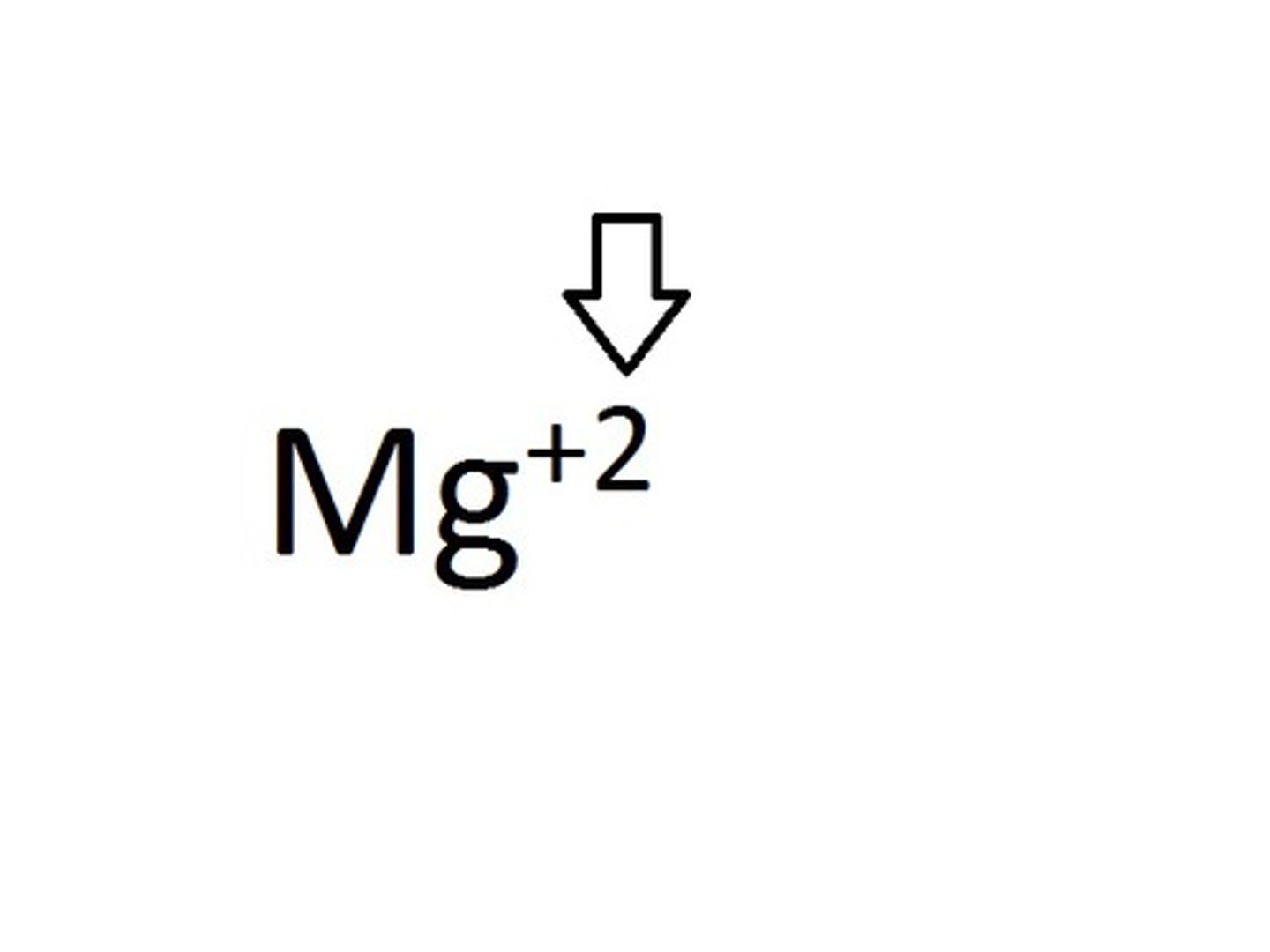
ion
An atom or group of atoms that has a positive or negative charge.
anion
an ion with a negative charge
cation
an ion with a positive charge
isotope
Atoms of the same element with different numbers of neutrons