Electrolysis of aqueous solutions
1/3
Earn XP
Description and Tags
https://www.youtube.com/watch?v=6WjC_Vi4roA&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=16
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
What are aqueous solutions
They are solutions dissolved in water
Water molecules ionise (splt) forming hydrogen ions and hydroxide ions.

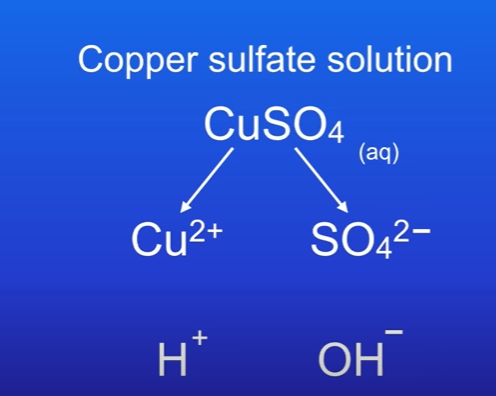
Explain the electrolysis of copper sulfate (aq)
Contains Cu2+ and SO4 2-.
Because its dissolved in water, we need to also consider hydrogen ion H+ and hydroxide ion OH-
At the cathode 2 different positive ions will be attracted, H+ and Cu2+.
To see which ion will be reduced at the cathode, we must look at reactivity series.
Hydrogen is produced at the cathode if the metal is more reactive than hydrogen.
Copper is less reactive so it will be reduced at the cathode.
At the anode, oxygen gas is made.
Its important that electrodes do not react with chemicals made in electrolysis.
Scientists say that electrodes are inert , in other words do not react. Platinum is used as inert as it is unreactive.
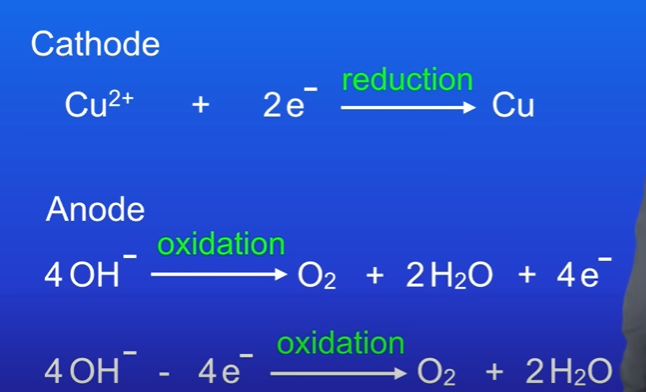
Whats a key fact regarding hydrogen and cathode
Hydrogen is produced at the cathode if the metal is more reactive than hydrogen.
What metal is the electrode made of
Platinum, as it is unreactive and we do not want the electrodes to react w chemicals being made during electrolysis.