NMR Spectroscopy
1/133
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
134 Terms
What can spectroscopy tell us?
Allows us to identify different substances that may look the same at surface level
Allows us to identify impurities within a substance
Tells us about the process of chemical synthesis of a substance
How does NMR work?
finds the structures of complex molecules using a magnetic field.
nuclei of an atom give off a signal when in the NMR machine
the signal depends on what the atom is bonded to
Why is NMR a good technique?
only a very small sample is needed.
the sample is not destroyed or used up in NMR
NMR can only analyse…
Nuclei with odd mass numbers
E.g. 1H, 13C
Why can NMR only analyse certain nuclei and not others?
Nuclei with odd mass numbers have a quantum mechanical property called ‘spin’
» this gives the nuclei magnetic properties
» so these nuclei are NMR visible
Which common nuclei are NMR invisible?
12C, 14N, 16O
What concept is the fundamental basis of NMR?
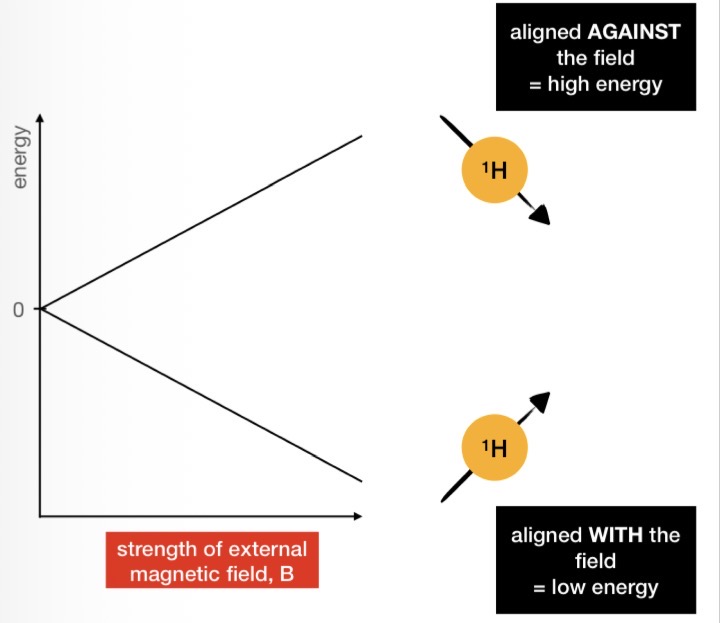
The Zeeman Effect
In the presence of a magnetic field (e.g. inside an NMR machine), the spins of nuclei split into 2 energy levels
Low energy = ‘spin up’ = aligned WITH the field
High energy = ‘spin down’ = aligned AGAINST the field
What gives rise to NMR signals?
What 2 things does the transition frequency between the low and high energy states depend on?
The nuclei transitioning between low and high energy levels
Frequency of a transition = frequency of radiation required for the nucleus to move between the 2 energy states
Transition frequency depends on:
Strength of the magnetic field
Stronger magnetic field = greater difference in energy between high and low energy levels = higher transition frequency = greater NMR signal
Gyromagnetic ratio
Tells us how magnetic a nucleus is
Higher gyromagnetic ratio = nucleus has a stronger interaction with the magnetic field (e.g. NMR spectrum) = larger difference in energy between the low and high levels = higher transition frequency = stronger NMR signal
Chemical Shifts
How does chemical shift occur?
Electrons around the nucleus can create opposing magnetic fields
This can shield the magnetic nucleus from the external magnetic field ie. The NMR machine
So nuclei feel the magnetic field less
More e- around the nucleus = more shielding = weaker magnetic field felt by nucleus = lower transition frequency = position of signal is more to the right
(vice-versa for less shielding)
Chemical Shift
What does chemical shift tell us?
Tells us how shielded or deshielded a nucleus is compared to a reference compound
The reference compound is TMS
Chemical Shift
What equation is used to calculate chemical shifts?
vobs = observed resonance frequency of sample nuclei
vref = resonance frequency of the reference compound (TMS)
The factor of x106 converts this ratio into parts per million (ppm) — which is the chemical shift scale
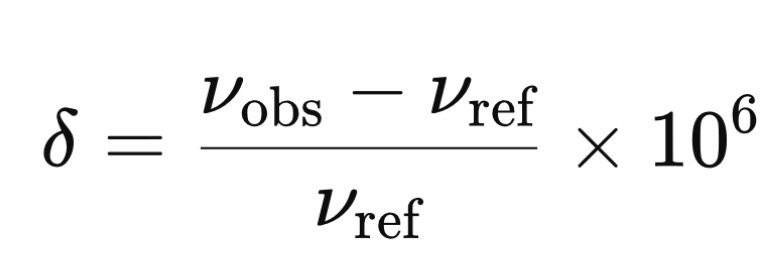
What is TMS?
A chemical used as the reference point to calculate chemical shifts
Given the value of 0 by definition
Why is TMS used to calibrate the spectrum?
Electroposiitve silicon has a shielding effect - so It’s signal is away from all others and doesn’t overlap with peaks of interest
Symmetric molecule - so only gives one signal so doesn't get in the way
Inert so doesn't react with the compound
Low boiling point so easy to remove, doesn't contaminate sample permanently
Non-toxic
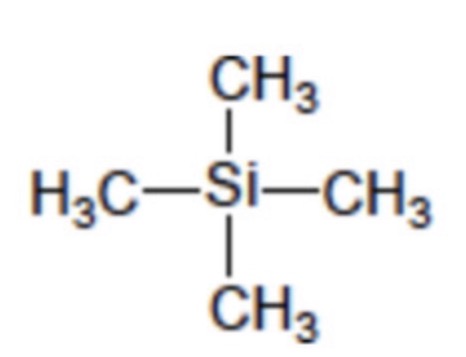
Give the structural formula for TMS
Si(CH3)4
Chemical Shift
Which symbol are chemical shifts represented by?
Delta - δ
Chemical Shifts
When there is less shielding, the resonance frequency (signal on the NMR) moves…
To the LHS
Chemical Shifts
When there is more shielding, the resonance frequency (signal on the NMR) moves…
To the RHS
Chemical Shifts
What can cause less shielding of the nucleus?
Electron withdrawing groups e.g. F, Br, I
Decrease the e- density around the nucleus
Deshields the nucleus
Resonance frequency more to the LHS
So larger chemical shifts in ppm
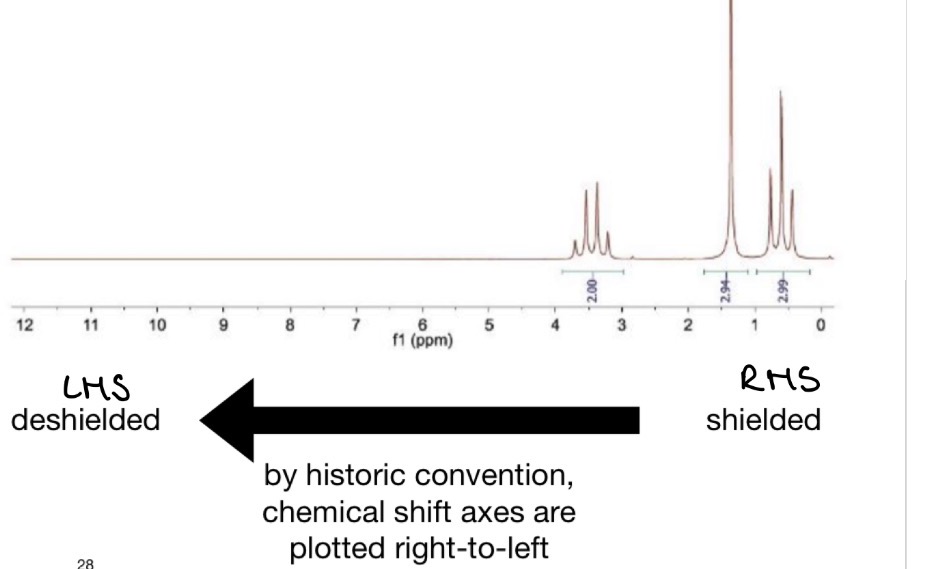
Chemical Shifts
What causes a larger chemical shift?
Nearby electronegative atoms = e- withdrawing = less shielding = resonance frequency shifts to the LHS = larger chemical shift
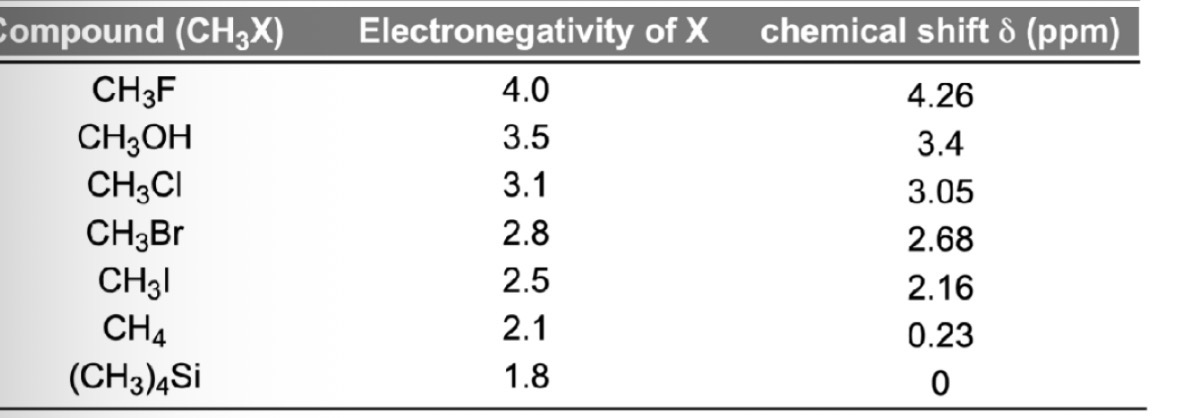
Chemical Shifts
Aromatic molecules and chemical shifts
Aromatic rings have delocalised π-electrons
In a magnetic field, π-electrons move in a circular motion → this is called the ring current
The ring current induces a local magnetic field that:
Opposes the external magnetic field (B0) inside the ring → so nuclei inside the ring are shielded
Reinforces the external magnetic force (B0) outside the ring → so nuclei outside the ring are deshielded e.g. aromatic Hs
Aromatic hydrogens are outside the ring = so are deshielded = feel stronger magnetic field
So resonance frequency appears to the LHS on NMR spectrum = greater chemical shift
Typical chemical shift: 6.5 – 8.5 ppm
E.g. benzene shows one signal at ~7.3 ppm
Chemical Shifts
Hydrogen bonding and chemical shifts
Labile protons form hydrogen bonds e.g. -OH or -NH
Hydrogen bonding causes deshielding of these protons
Because H-bonds constantly form and break, deshielding strength varies
This means labile protons can be observed over a broad range of chemical shifts
Solvent, acidity, concentration, and temperature also affect their chemical shifts
This makes the chemical shifts of labile protons hard to predict
-OH and -NH therefore appear as broad peaks in NMR (often labelled “br”)
Chemical Shifts
How do -OH and -NH proteins appear on the NMR soectrum
As broad peaks
Do not have integration, and usually marked as ‘br’
Chemical Shifts
How can -OH and -NH be identified experimentally?
Identified by D₂O exchange experiment (“D₂O shake”)
Labile proton replaced by deuterium = signal disappears
Chemical Shifts
Common 1H chemical shifts to memorise (check these values idk if right)
-OH = 3.9 - 9.0ppm
-NH = 5.5 - 8.5ppm
O=C-H = 9.5ppm
Benzene-H = 6 - 8.5ppm
O-C-H and X-C-H = 2.8 - 4.9ppm
N-C-H, S-C-H, O-C-C-H, C=C-C-H, C6H6-CH2-H = 1.8 - 3.1ppm
-CH, -CH2, -CH3 = 0 - 1.9ppm
What is an environment?
what the atoms are bonded to.
number of environments = number of signals
If hydrogens are in the same environment they are…
Chemically equivalent
All hydrogens on the same carbon…
are in the same environment
so are chemically equivalent
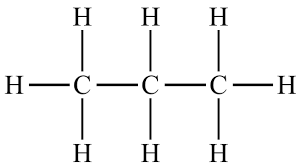
How many environments are in propane?
2
Blue: each H is bonded to a C, which is bonded to H2, CH2, CH3
Green: each H is bonded to a C, which is bonded to H, CH3, CH3
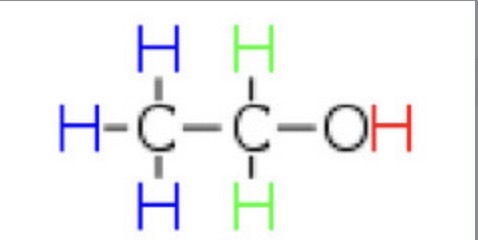
Why are there 3 peaks in the NMR spectrum for ethanol?
There are 3 different environments:
Blue: H is bonded to a C, which is bonded to a H, CH2, OH
Green: H is bonded to a C, which is bonded to CH3, OH
Red: H is bonded to O, which is bonded to CH2, CH3
How many signals would you see on the H-NMR spectra for this compound?
3
Symmetrical H…
Are in the same environment
How many environments in this molecule
There is one line of symmetry
2 environments
Another way of looking at H in the same environment…
same distance away from a particular atom
e.g. all the H in cyclohexane are the same number of H atoms away from the top C
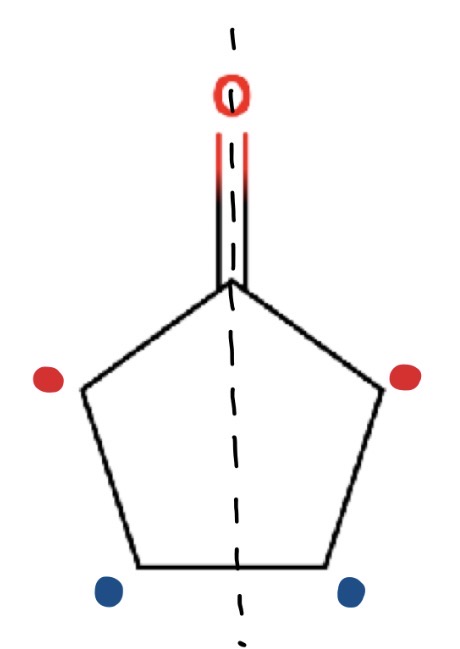
How many environments in this molecule
There are 2 lines of symmetry
2 environments
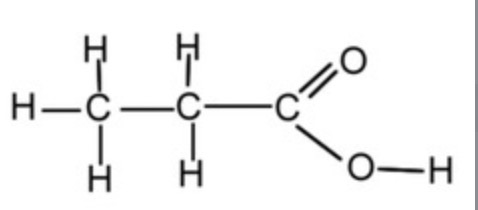
How many peaks would you see in the proton NMR of the following molecule: CH2ClCH2CH2CH2Cl
2 environments
2 peaks
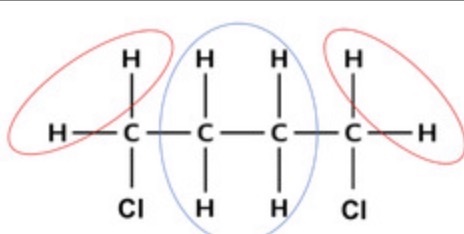
Predict how many peaks there would be in an H-NMR spectrum of these molecules:
Cyclohexane
Methylcyclohexane
1 peak
5 peaks
How many peaks/environments in this molecule?
4 environments
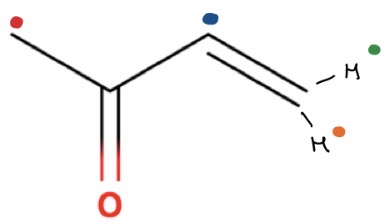
How many peaks/molecules in this molecule
4 environments
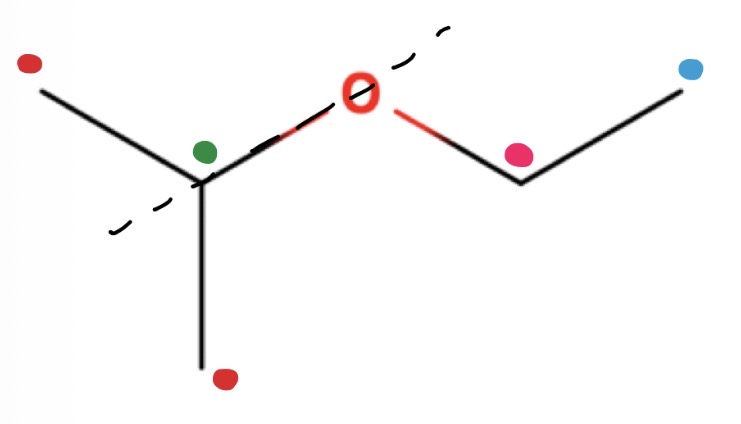
Counting environments top top
See if any lines of symmetry
Draw in symmetry line
All Hs on either side of symmetry line are in the same environment
Which 2 rare isotopes are NMR-active?
Why do both have very low sensitivity?
13C and 15N
Both have very low abundance (13C: 1.1%, 15C: 0.37%)
Why must samples be dissolved in solvents free of 1H atoms?
What is an exception to this?
Solvents with 1H will produce a signal (residual peaks) in the NMR and interfere
Deuterium (D) which is 2H — even mass number so does not produce a signal in the 1H NMR
So deuterated solvents are NMR-invisible
What is D?
Deuterium
Written as D or 2H e.g. CDCl3
Why can samples be dissolved in solvents which contain D?
Deuterium (2H) has even mass number so does not produce a signal in the 1H NMR
Common solvents used in NMR
CDCl3 - deuterated chloroform
D2O
Deuterated DMSO
How does NMR work?
Samples are dissolved in a deuterated solvent e.g. CDCl3 and placed in a strong magnetic field
A small amount of TMS is added to calibrate the machine
A radiofrequency pulse is applied to the sample, changing the nuclear spin of the nuclei
The energy associated with these transitions is recorded by the detector
Generates a spectrum for analysis
Summary of the different info in an NMR spectrum
Number of peaks
Peak integration (area of peaks)
Chemical shift (position of peaks)
Shape of peaks (multiplicity/splitting/J coupling)
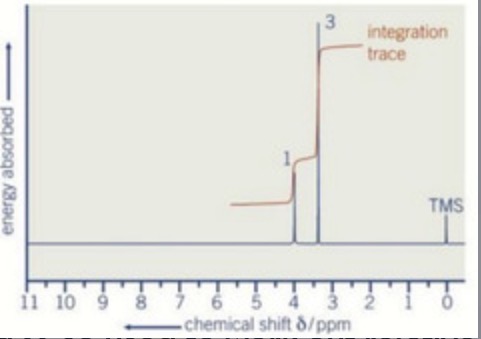
What is the y-axis on an NMR spectrum?
Energy absorbed (used to work out integration)
What is the x-axis on an NMR spectrum?
Chemical shift
Peak Integration
What is it?
The number of 1Hs in each environment in the molecule
E.g. CH3-CH2-OH has an integration ratio of 3:2:1
Peak Integration
How is the peak integration shown on an NMR spectrum?
As ratios under each peak
As a whole number above each peak
Shown graphically
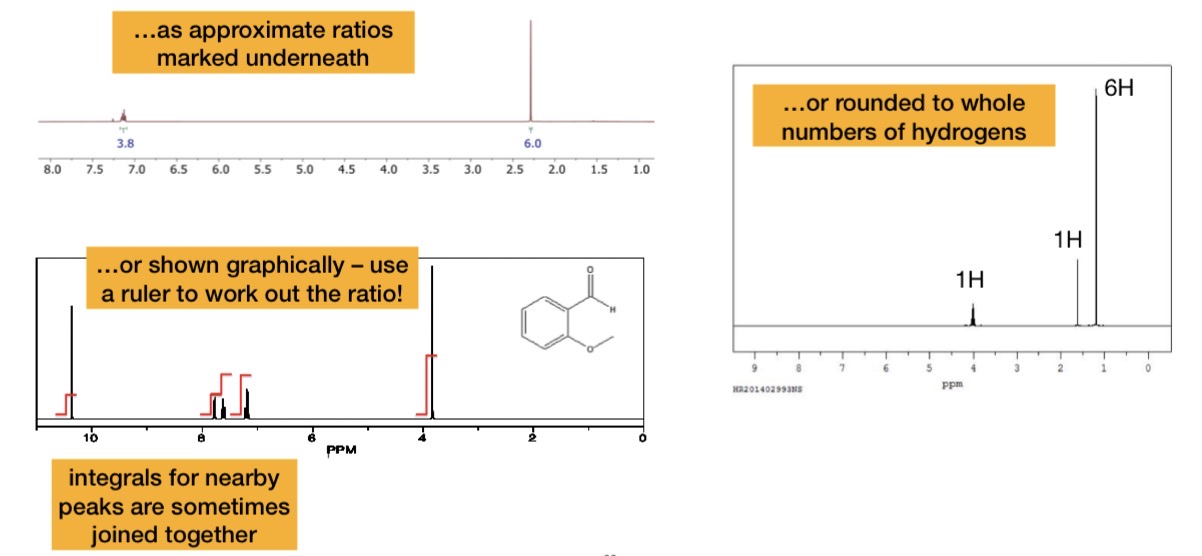
Peak Integration
For each of the following molecules, state the number of peaks and their peak integrals
A. 2 peaks, 3:1
B. 4 peaks, 3:1:2:3
C. 5 peaks, 3:1:2:2:3
D. 4 peaks, 6:1:2:3
Peak Integration
How many peaks are present and what are the peak integrals?
2 peaks
3:2
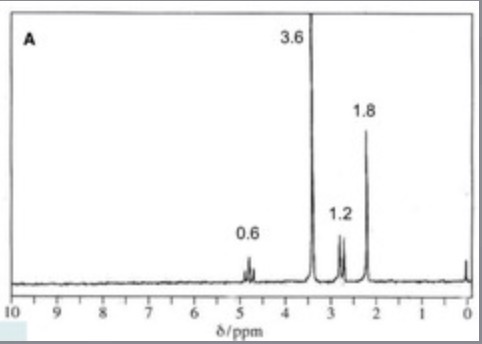
Peak Integration
What is the integration ratio on this spectrum
0.6 : 3.6 : 1.2 : 1.8
=
1 : 6 : 2 : 3
(divide by smallest number 0.6)
Multiplicity / J Coupling
Why do signals on the NMR spectrum split?
Hydrogen atoms adjacent to each other interfere with the signal
Multiplicity / J Coupling
How to work out how much a peak splits
Number of Hs bonded to adjacent C + 1
Multiplicity / J Coupling
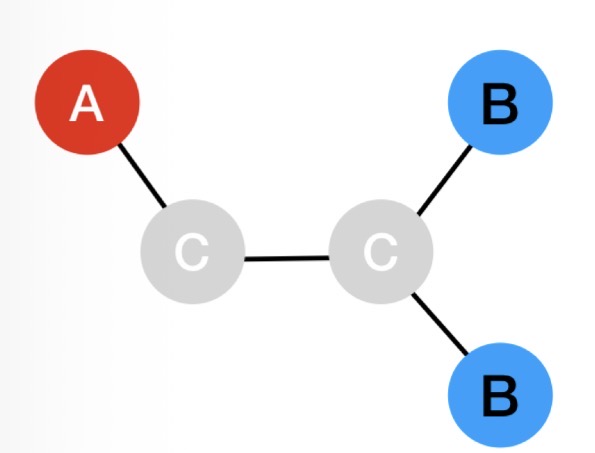
Explain why both HA and HB would split into a doublet:
HA- C - C - HB
The signal for peak HA is split into a doublet, depending on whether the spin of the adjacent HB up or down
The signal for peak HB is split into a doublet, depending on whether the spin of the adjacent HA up or down
Multiplicity / J Coupling
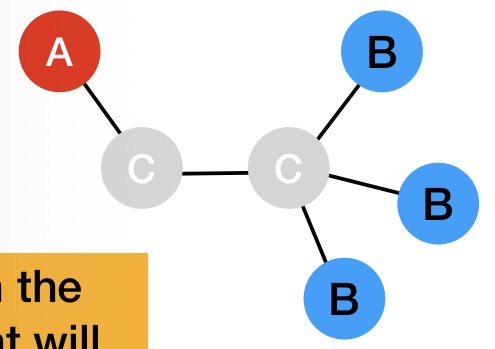
Explain what the multiplicity will be like for HA in the following molecule
2 adjacent Hs = triplet
Both HBs can spin up (1 way this can happen)
1 HB can spin up and the other HB can spin down (2 ways this can happen)
Both HBs can spin down (1 way this can happen)
So HA forms a 1:2:1 triplet
Multiplicity / J Coupling
Explain what the multiplicity will be like for HA in the following molecule
1:3:3:1 quartet
Multiplicity / J Coupling
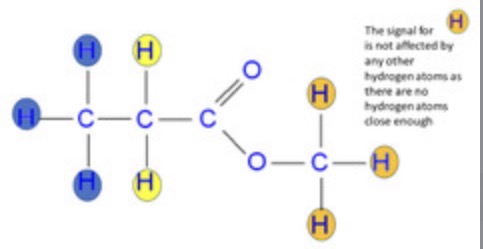
Work out the degree of multiplicity for this molecule
• Blue: 3 peaks with ratio 1:2:1
• Yellow: 4 peaks with ratio 1:3:3:1
• Orange: 1 peak, no splitting
Multiplicity / J Coupling
Name all the types of splitting patterns
• singlet — no H on adjacent C
• doublet — 1 H on adjacent C
• triplet — 2 H on adjacent C
• quartet — 3 H on adjacent C
Multiplicity / J Coupling
How many H environments?
What is the splitting pattern?
2 environments
Green: triplet with ratio 1:2:1
Orange: quartet with ratio 1:3:3:1
Multiplicity / J Coupling
State the multiplicity of HA in this molecule
3 environments
HA is adjacent to both HB and HC which are in different environments
HB can either spin up or down - 2 options
HC can either spin up or down - 2 options
2 × 2 = 4
So HA forms a doublet of doublets (d) with the ratio 1:1:1:1
Multiplicity / J Coupling
When does a nucleus split into a doublet of doublets?
When the H is adjacent to 2 Hs in different environments
Forms 4 peaks which are NOT evenly spaced (unlike a quartet)
Multiplicity / J Coupling
If a Q asks you to state the number of coupled spins
Just the number of adjacent Hs in different environments
CH3-NH2
Even though the chemical shift for CH3 says it is 0.7-1.2ppm on the data sheet, why may the peak for CH3 hydrogens be around 1.4ppm?
If hydrogen atoms are close to electronegative atoms, their shift on the spectrum is higher than the data sheet predicts
H atoms in CH3 are adjacent to N
Predict the chemical shift for the hydrogens in each environment. (2-bromo-2-methylbutane)
Red: 0.7–1.2 ppm
Blue: 2.1–2.6 ppm
Green: 0.7–1.2 ppm
2-bromo-2-methylbutane
Number of signals, Integration, J-coupling, Chemical shifts
Number of signals: 3
Integration: 3 : 2 : 6
J-coupling: t, q, s
Chemical shifts: 0.7–1.2 ppm, 1.2–1.4 ppm, 0.7–1.2 ppm
Methylpropene
Number of signals, Integration, J-coupling, Chemical shifts
Number of signals: 2
Integration: 3 : 1
J-coupling: s, s
Chemical shifts: 0.7–1.2 ppm, 4.5–6.0 ppm
Ethyl propanoate
Number of signals, Integration, J-coupling, Chemical shifts
Number of signals: 4
Integration: 3 : 2 : 2 : 3
J-coupling: t, q, q, t
Chemical shifts: 0.7–1.2 ppm, 0.7–1.2 ppm, 2.1–2.6 ppm, 3.7–4.1 ppm
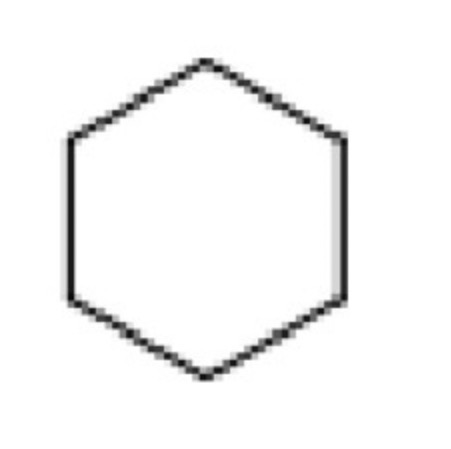
Predict the number of signals, Integration, J-coupling, Chemical shifts of: Cyclohexane
Number of signals: 1
Integration: n/a (as no other peaks so nothing to compare intensity to)
J-coupling: singlet
Chemical shift: 1.2–1.4 ppm
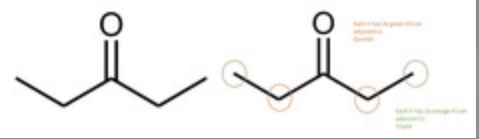
Predict the Number of signals, Integration, J-coupling, Chemical shifts of: Pentan-3-one
Number of signals: 2
Integration: 3 : 2
J-coupling: triplet and quartet
Chemical shifts: 0.7–1.2 ppm and 2.1–2.6 ppm
Steps for identifying compounds from their HNMR spectrum
Look at number of peaks/environments
Put chemical shifts in table in descending order.
Put relative intensities in table.
Put j-coupling in table with ratio
Draw structure fragments from table.
Join fragments using splitting pattern (fragments that overlap mean they are next to each other; find end pieces first).
Check molecular formula and splitting.
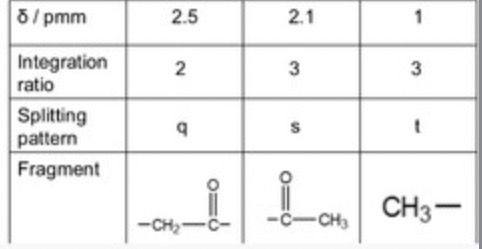
A singlet with an intensity of 1 is usually…
–OH
If you see an NMR with a peak that is a quartet, and a peak that is a triplet it is usually….
CH2–CH3 (but always double check)
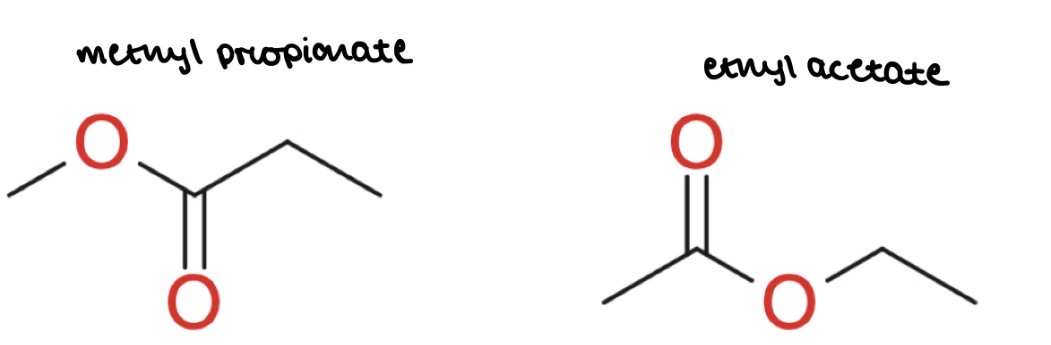
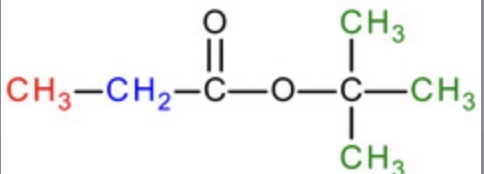
How would you use NMR to distinguish ethyl acetate from methyl propionate
Both have 3 environments
Both have an integration of 3:2:3
Both have j-coupling of s, q, t
However, they have different chemical shifts:
ethyl acetate:
CH2 bonded to O = more shielding = quartet more towards RHS
CH3 bonded to C=O = less shielding = singlet towards LHS
methyl propionate:
CH3 bonded to O = more shielding = singlet towards RHS
CH2 bonded to C=O = less shielding = quartet towards RHS
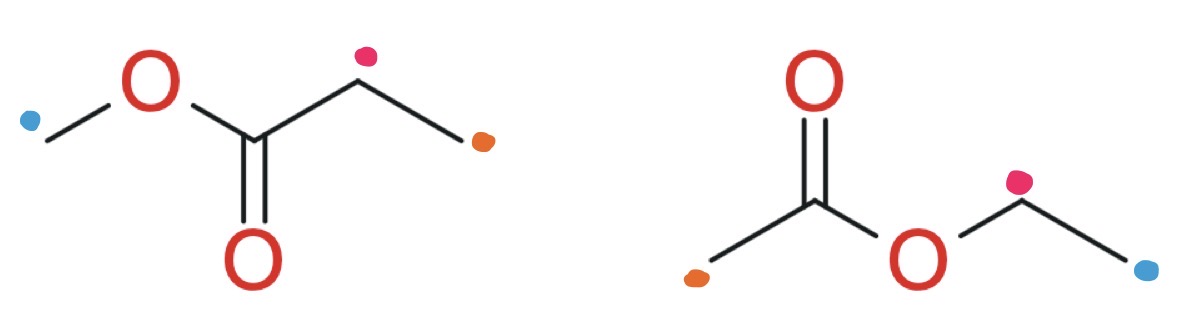
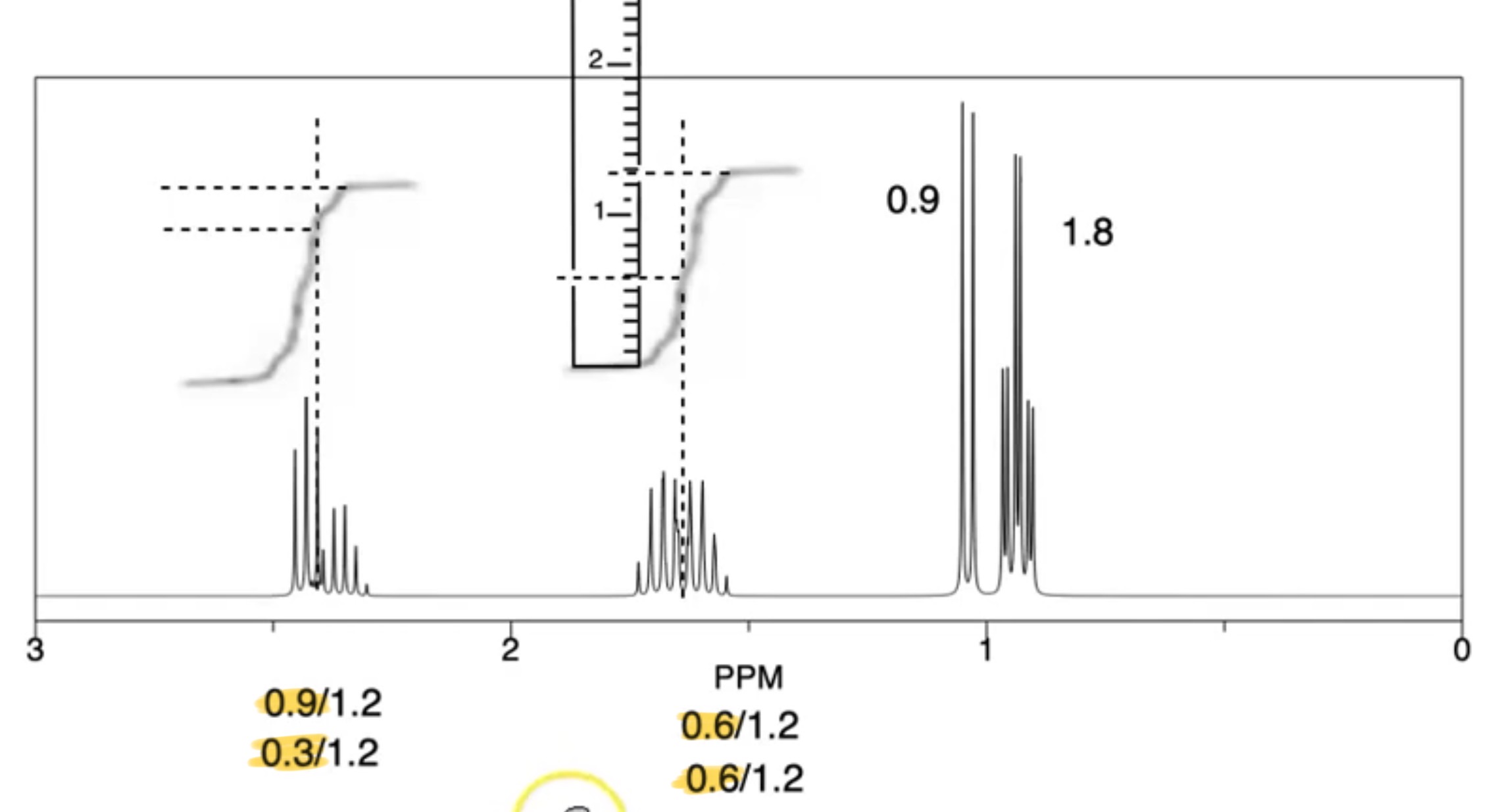
How do you work out the integration ratio if graphically shown on the spectra
Use a ruler to measure the length of each curve from bottom to top
That gives you the integration ratio
Use this to work out number of Hs in each environment
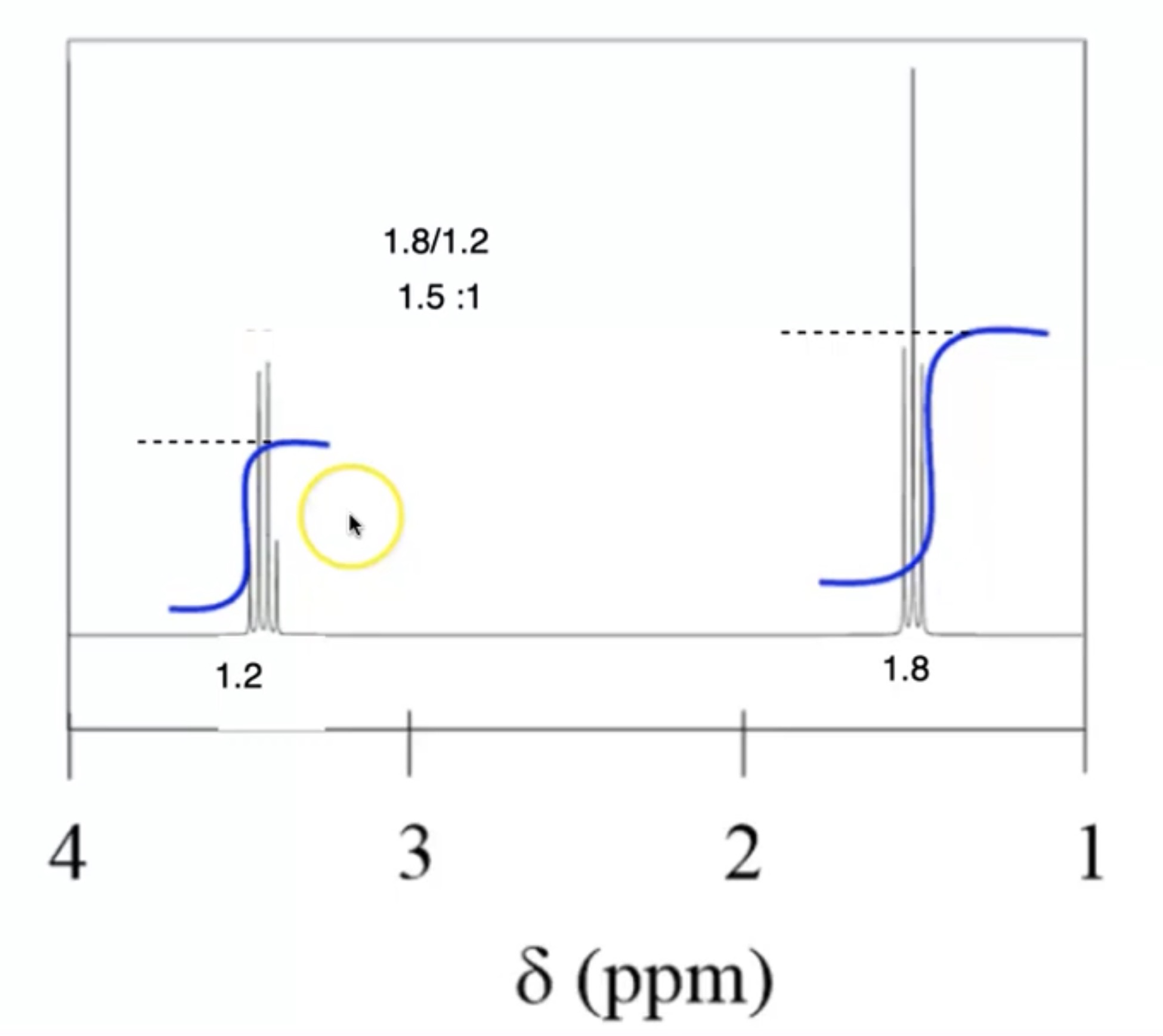
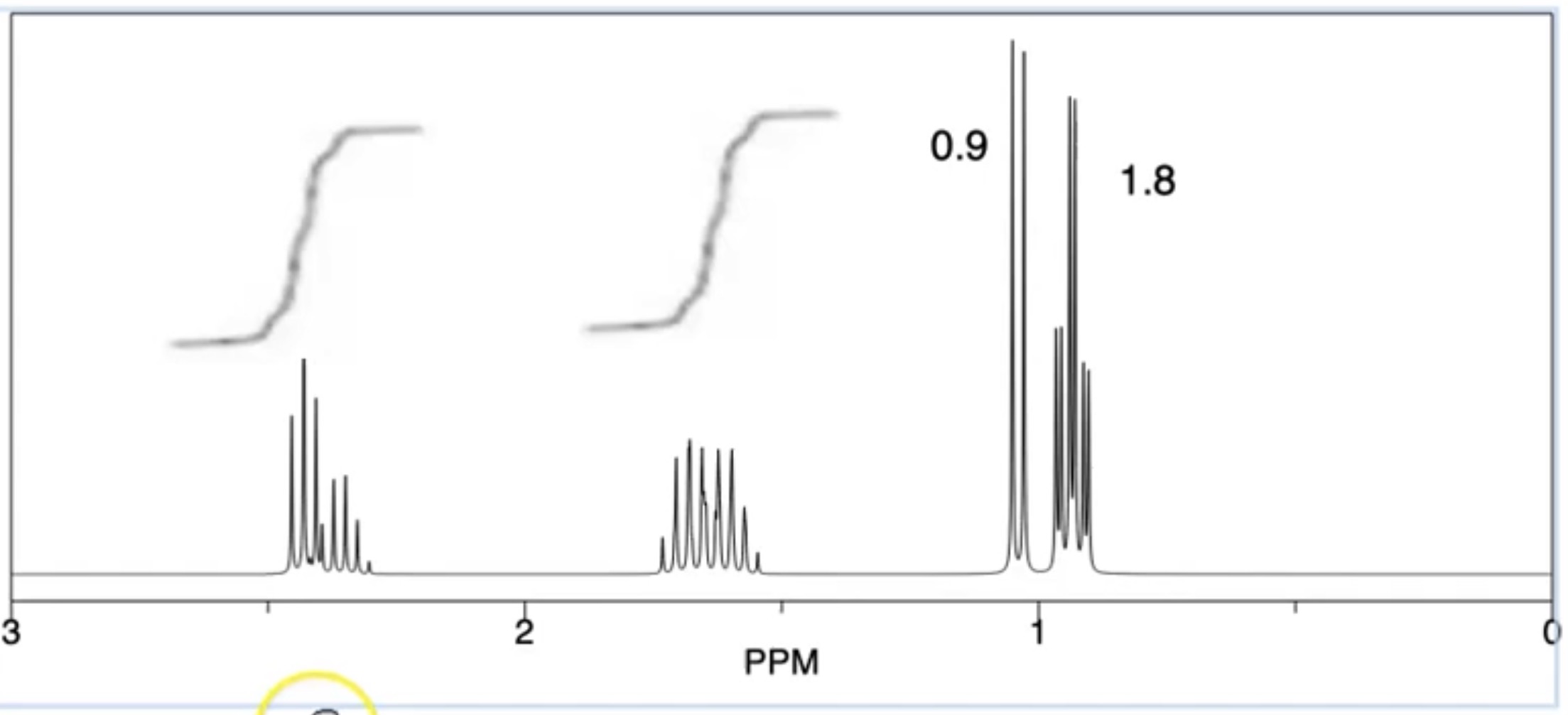
In this instance, there is a triplet overlapping with a quartet and 2 overlapping multipliers. How would you work out the integration for each.
Split peak into triplet and quartet
Measure each with a ruler
That is the ratio for each
Divide all numbers by smallest number to get actual number of Ha in each environment
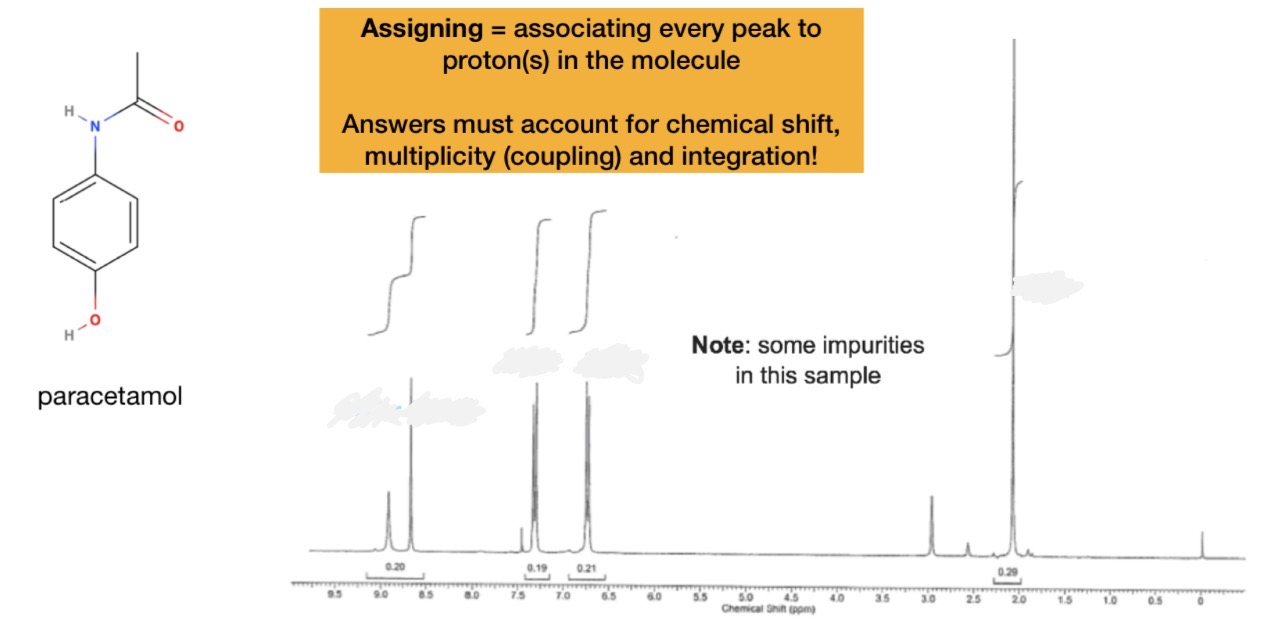
Assign this spectrum of paracetamol
Calculate integration, splitting and chemical shift for each environment in the molecule
Calculate integration, splitting and chemical shift for each signal on spectrum
Match them up
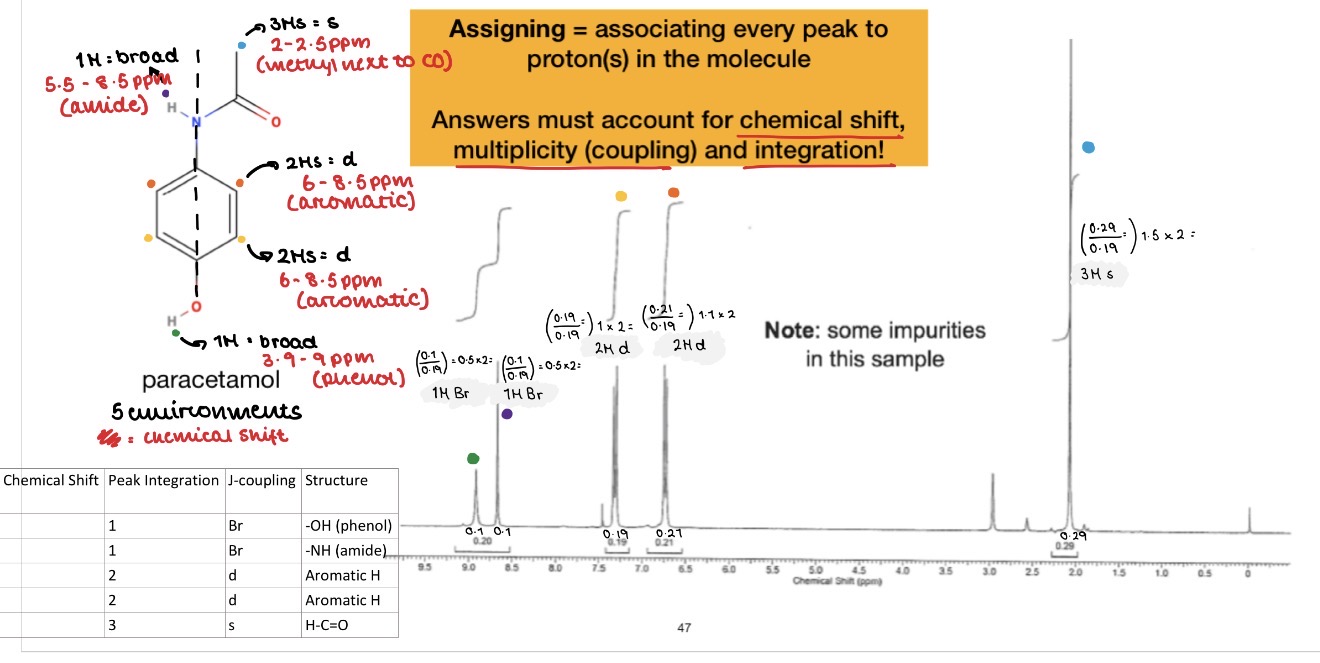
A molecule has the molecular formula C4H8O. Use the spectra to work out its structure.
• 3 peaks = 3 environments
• Peak integration 2 : 3 : 3
• Peak at 2.1 ppm is CH3–C=O. It is a singlet because adjacent C has no Hs
• Other peaks at 2.5 and 1 are CH3–CH2–C=O. They have a splitting pattern of triplet and quartet which means they are a CH3 and CH2 bonded together
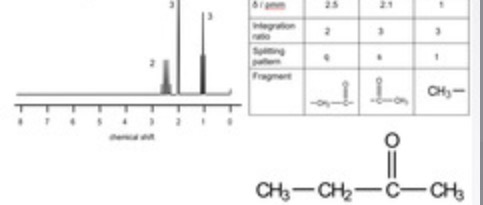
The 1H NMR spectrum of C6H12O2 is shown. Deduce the structure of the compound.
5 peaks = 5 environments
Peak integration is 2 : 3 : 2 : 2 : 3
Peak at 3.2 ppm is –O–CH3. It is a singlet as no adjacent C with Hs
Peak at 1 ppm is –CH3. It is a triplet so it is next to CH2
Peaks at 2.6 ppm and 2.5 ppm are –CH2COCH2–
Peak at 2.6 ppm triplet, so attached to C with 2 Hs
Peak at 2.5 ppm is a quartet, so attached to C with 3 Hs
Molecule is CH3CH2COCH2CH2OCH3
How are carbon environments commonly referred to?
1° = primary = 1 bond to heavy atoms = X-CH3
2° = secondary = 2 bonds to heavy atoms = X-CH2-X
3° = tertiary = 3 bond to heavy atoms = CX3H
4° = quaternary = 4 bond to heavy atoms = CX4
How many carbon environments are there?
6
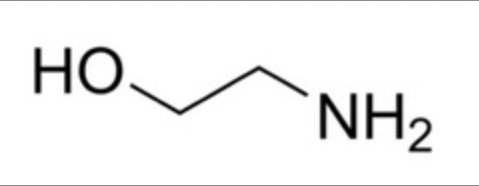
How many carbon environments are there?
2
Rule for number of carbon environments
If Hs are in different environments, the Cs they are bonded to will be in different environments
C-NMR is done with…
Carbon-13 (13C)
Why do we not get peaks with carbon-12 atoms?
12C atoms don't have spin as they have an even mass number
How many peaks in the 13C NMR of butane?
2
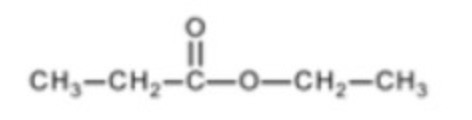
How many carbon environments?
5
How many carbon environments?
5
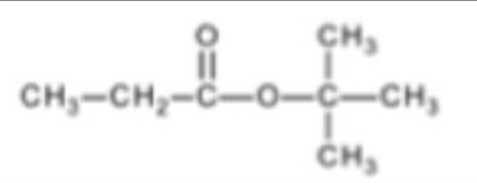
How many carbon environments in but-2-ene?
2
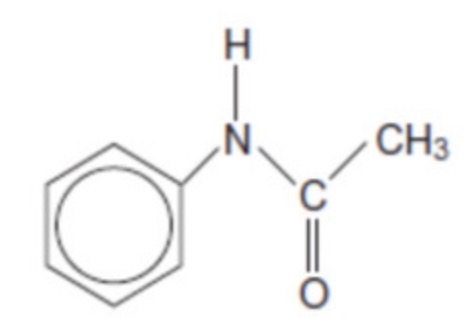
The structure of N-phenylethanamide is shown. Determine the number of peaks in the 13C NMR spectrum
6
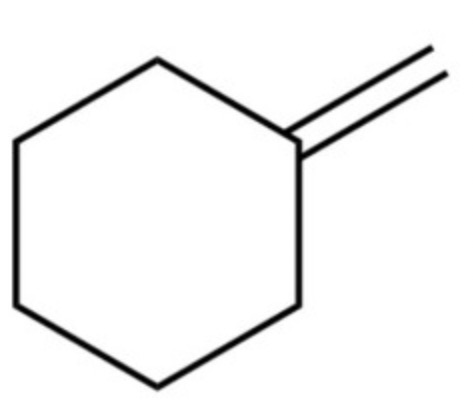
How many peaks in the 13C NMR spectrum?
5
In 13C NMR we only have to look at…
Number of peaks / environments
Chemical shift
» NO integration or scalar coupling considered
Scalar coupling in 13C NMR
The chance of having 2 13C atoms next to each other is very small » so very small chance of having 13C coupling to other 13C atoms
Scalar coupling can occur between 13C and 1H » however this is purposefully supressed via decoupling to make the spectra easier to interpret
Why do we experimentally suppress scalar coupling between 13C and 1H in 13C NMR?
13C signals are naturally weak (only ~1.1% abundance).
Coupling to multiple hydrogens would split signals and spread them out, reducing sensitivity.
Removing coupling gives one sharp, strong peak per unique carbon — easy to assign
13C chemical shifts to memorise
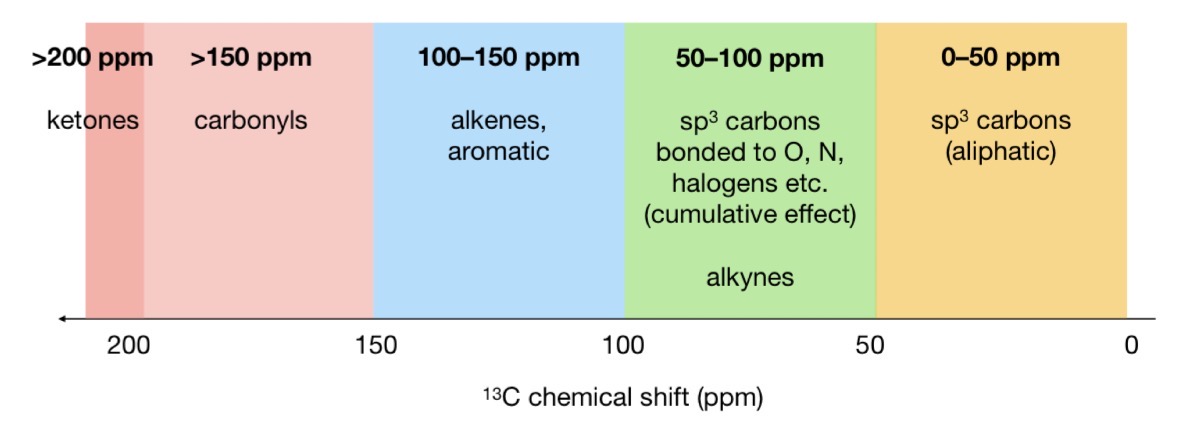
If an sp3 C is bonded to a more e- withdrawing group…
Chemical shift is larger and further to the left
Quaternary Cs in 13C NMR
quaternary carbons (C not bonded to any Hs) are usually less intense than other signals » have a shorter peak
If there is a very short peak on 13C NMR…
We can assume it is a 4° C
Can use this to work out which atoms have Cs or Hs attached e.g. in aromatic rings
E.g. the green Cs are quaternary so will have a less intense peak on the NMR
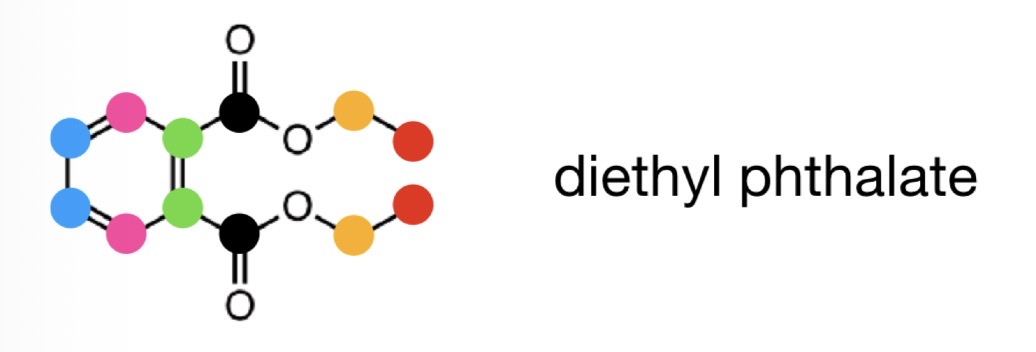
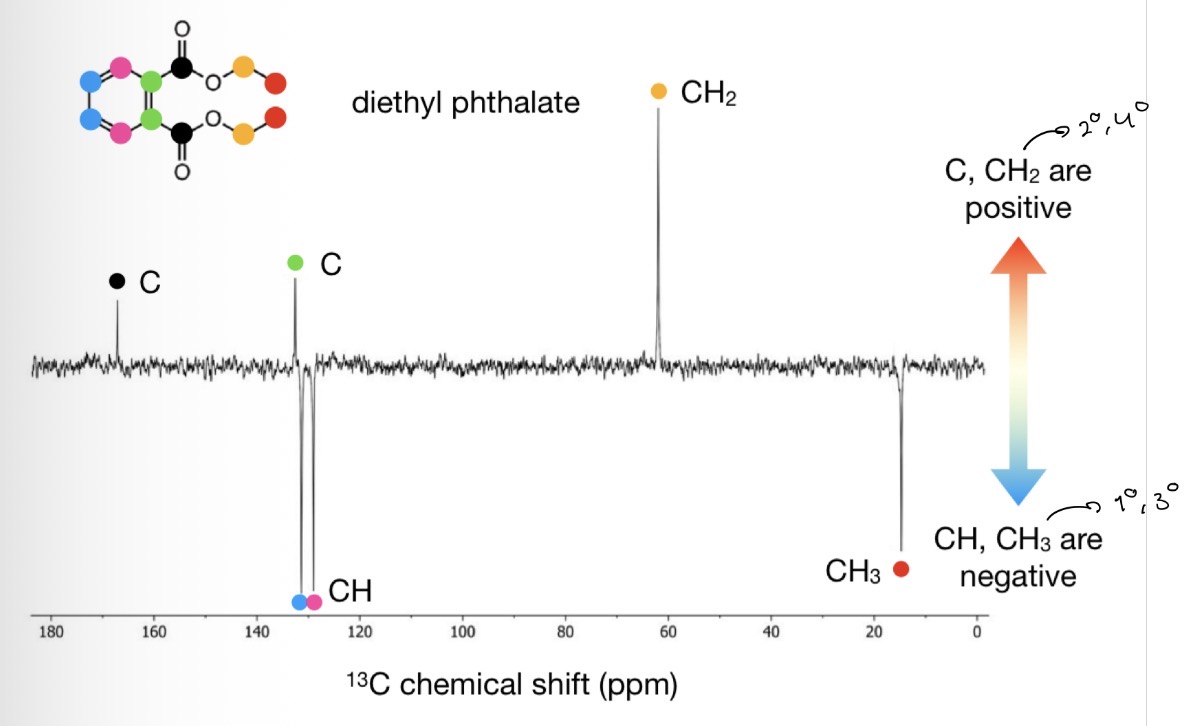
What is the Attached Proton Test (APS)?
A variant of a 13C NMR experiment
In APR spectra 1° and 3° Cs appear with the opposite sign to 2° and 4° Cs
E.g. in the example, C and CH2 are positive and CH and CH3 are negative
We can work out which is positive and which is negative by locating the 4° C (peak with the lowest intensity)