lec 22 - dialysis (yang)
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
background: kidney
bean shaped structure
each kidney weights ~150 grams
consists of cortex (outer) and medulla (inner)
millions of nephrons
each nephron consists of
glomerular apparatus
proximal tubule
loop of henle
distal tubule
collecting ducts
types of nephrons
cortical
intermedial
juxtamedullary

renal clearance depends on…
GFR
tubular reabsorption
tubular secretion
glomerular filtration (GFR)
passive filtration of the blood as blood flows through the glomeruli of kidney
extent to which a drug is filtered depends on:
molecular size
protein binding
ionization
polarity
kidney function
tubular reabsorption
some drugs may be reabsorbed after being filtered out of the blood
thus, CLR may be smaller than expected (when considering only filtration and CLR = GFR * fu)
if a drug is “completely” reabsorbed after filtration and NO active secretion takes place, renal CL will be limited to the amount of drug that leaves the kidney as the urine flows into the bladder
tubular secretion
can increase the CLR by actively secreting the drug (as opposed to only passive diffusion in GFR)
rate of secretion depends on the transporter
if transporter is slow, secretion will depend on fraction unbound
very efficient active transport (an absence of any reabsorption) → max renal CL
GFR and urine output
GFR
kidneys receive 20% cardiac output
5 L/min (cardiac output) * 0.2 = 1 L/min
60% of this volume is plasma
1 L/min * 0.6 = 0.6 L/min
20% is filtered (passes thru glomerular barriers)
600 mL/min * 0.2 = 120 mL/min just GFR
urine output
rate of excretion = rate of filtration - rate of absorption + rate of secretion = 120 mL/min
120 mL/min * 1440 min/day = 172.8 L day
120 mL/min - reabsorption (no secretion) = 172.8 L/day - 99% = 1.78 L/day * 0.01 = 1.73 L/day
chronic kidney disease (CKD)
definition = abnormalities of kidney structure or function present for over 3 months that have implications on health
diagnosis
estimated GFR decreases to <60 mL/min/1.73m2
1 or more markers of kidney damage e.g. albuminuria, histologically detected abnormalities
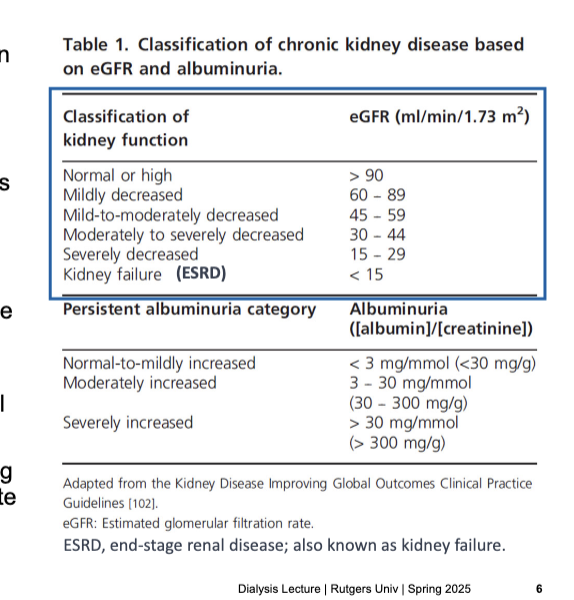
progressive disorder and stages classified based on eGFR and albuminuria
10-15% of the population with some degree of CDK and over 1 million pts worldwide now receive renal replacement therapy to treat kidney failure
heightened risk of medication-related problems → dosing errors in pts with CKD still occur at alarming rate
classification of kidney function → eGFR (mL/min/1.73 m2)
normal or high → >90
mildly decreased → 60-89
mild-to-moderately decreased → 45-59
moderately to severely decreased → 30-44
severely decreased → 15-29
kidney faillure (ESRD) → <15
PK changes in chronic kidney disease (CKD)
drug absorption and bioavailability
delayed gastric emptying and intestinal motility → impact on Tmax and C max of drugs
high gastric pH
excess urea in the saliva transformed to ammonia by gastric ureas → raises pH
resulting alkalization affects the ionization and dissolution of drugs
drug bioavailability more variable in pts with impaired kidney function
uremia (increased urea) decreases GI absorption of drugs and alters 1st pass metabolism
distribution
altered volume of distribution (e.g. dehydration or muscle wasting)
altered plasma protein and tissue binding of drugs
metabolism
uremia slows the rate of phase I metabolism (reduction, oxidation, hydrolysis) and some phase II metabolism pathways
dependent on the kidney for the removal of drug metabolites from the body
complicated impact on drug metabolism including changes in the expression of several CYP enzymes (intestinal and hepatic) and transporters reported
elimination
renal CL depends on GFR, tubular reabsorption, and tubular secretion
decreased GFR → decreased renal CL → increased plasma half life
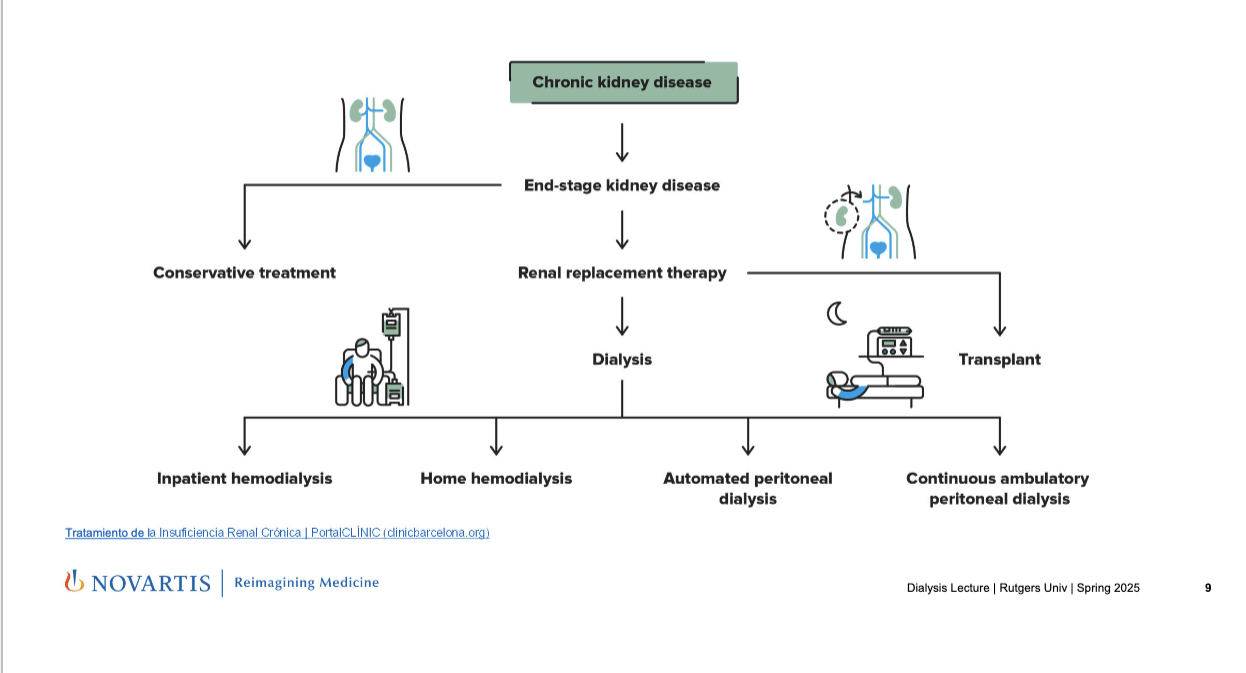
treatment options
CKD → end-stage kidney disease
→ conservative treatment: for early stages
→ renal replacement therapy
→ transplant
→ dialysis: mechanically replaces kidney function by removing waste and fluid
→ inpatient hemodialysis: done at dialysis center or hospital
→ home hemodialysis: pt or caregiver operates dialysis machine at home
→ automated peritoneal dialysis: done at night using a machine while the patient sleeps
→ continuous ambulatory peritoneal dialysis: done manually during the day WITHOUT a machine
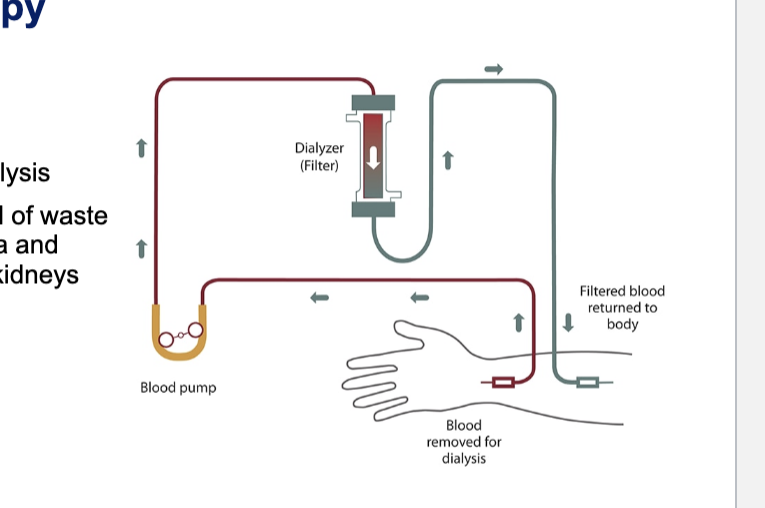
renal replacement therapy: hemodialysis
also known as haemodialysis or simply dialysis
achieves the extracorporeal removal of waste products such as creatinine and urea and free water from the blood when the kidneys are in a state of failure
diagram
blood is removed from the body
blood pump actively draws blood from the dialysis circuit → ensures constant, controlled flow rate
dialyzer (filter) = artificial kidney
blood flows thru semi-permeable membranes
waste products, excess ions, and fluid move across the membrane into the dialysate
filtered blood is returned to the body
renal replacement therapy: hemodialysis (more in depth?)
definition
process of removing heparinized blood (anticoagulant) from the body, passing thru a semi-permeable membrane on the opposite side of a dialysate
waste products and extra body fluid move from the blood → dialysate and is discarded; clean blood is then returned to the patients
for patients who are hemodynamically stable
vascular access
IV catheter, arteriovenous fistula
dialyzers
high flux (most common)
blood flow = ~500 mL/min
3-4 hour sessions
3 times a week
dialyzers (high flux)
artificial fibers that mimic kidney’s filtering function
polysulfone
polymethylmethacrylate
polyacrylonitrile
dialysate
fluid that is on the opposite side of the membrane from the blood
concurrent flow → dialysate moves in the opposite direction to blood → maximizes gradient and waste removal
flow rate = 500-800 mL/min
various solutes and anticoagulants
waste and fluid removal
diffusion → solutes like urea, creatinine, K+
ultrafiltration → removal of excess water via pressure gradient
concentration gradient against the dialyzer membrane (pull water out)
dialysis prescription
flow rate
duration of dialysis
dialyzer
measures of adequacy
urea reduction ratio (URR)
[(BUNpre - BUNpost)/BUNpre]*100
>70% is considered adequate (KDOQI guidelines)
Kt/V
Kt = dialyzer CL of urea
T = duration of dialysis
V = volume of blood cleared from urea
Goal = Kt/V >= 1.3
properties of a dialyzable drug
MW <5000 Da
Vd <1 L/kg (mostly stays in plasma)
protein binding <90% (only free drug is dialyzable)low lipid solubility (hydrophilic drugs stay in plasma)
![<ul><li><p>definition</p><ul><li><p>process of removing heparinized blood (anticoagulant) from the body, passing thru a semi-permeable membrane on the opposite side of a dialysate</p></li><li><p>waste products and extra body fluid move from the blood → dialysate and is discarded; clean blood is then returned to the patients</p></li><li><p>for patients who are hemodynamically stable</p></li></ul></li><li><p>vascular access</p><ul><li><p>IV catheter, arteriovenous fistula</p></li></ul></li><li><p>dialyzers</p><ul><li><p>high flux (most common)</p><ul><li><p>blood flow = ~500 mL/min</p></li><li><p>3-4 hour sessions</p></li><li><p>3 times a week</p></li></ul></li></ul></li><li><p>dialyzers (high flux)</p><ul><li><p>artificial fibers that mimic kidney’s filtering function</p><ul><li><p>polysulfone</p></li><li><p>polymethylmethacrylate</p></li><li><p>polyacrylonitrile</p></li></ul></li></ul></li><li><p>dialysate</p><ul><li><p>fluid that is on the opposite side of the membrane from the blood</p></li><li><p>concurrent flow → dialysate moves in the opposite direction to blood → maximizes gradient and waste removal</p></li><li><p>flow rate = 500-800 mL/min</p></li><li><p>various solutes and anticoagulants</p></li></ul></li><li><p>waste and fluid removal</p><ul><li><p>diffusion → solutes like urea, creatinine, K+</p></li><li><p>ultrafiltration → removal of <strong>excess water</strong> via <strong>pressure gradient</strong></p></li><li><p>concentration gradient against the dialyzer membrane (pull water out)</p></li></ul></li><li><p>dialysis prescription</p><ul><li><p>flow rate</p></li><li><p>duration of dialysis</p></li><li><p>dialyzer</p></li></ul></li><li><p>measures of adequacy</p><ul><li><p>urea reduction ratio (URR)</p><ul><li><p>[(BUN<sub>pre</sub> - BUN<sub>post</sub>)/BUN<sub>pre</sub>]*100</p></li><li><p><strong>>70%</strong> is considered adequate (KDOQI guidelines)</p></li></ul></li><li><p>Kt/V</p><ul><li><p>Kt = dialyzer CL of urea</p></li><li><p>T = duration of dialysis</p></li><li><p>V = volume of blood cleared from urea</p></li><li><p>Goal = Kt/V >=<strong> 1.3</strong></p></li></ul></li></ul></li><li><p>properties of a dialyzable drug</p><ul><li><p>MW <strong><5000 Da</strong></p></li><li><p>Vd <strong><1 L/kg</strong> (mostly stays in plasma)<br>protein binding <strong><90%</strong> (only free drug is dialyzable)</p></li><li><p>low lipid solubility (hydrophilic drugs stay in plasma)</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/eff66842-1caa-4c6e-993c-aa4d1e79ad15.png)
renal replacement therapy: peritoneal dialysis pt 1
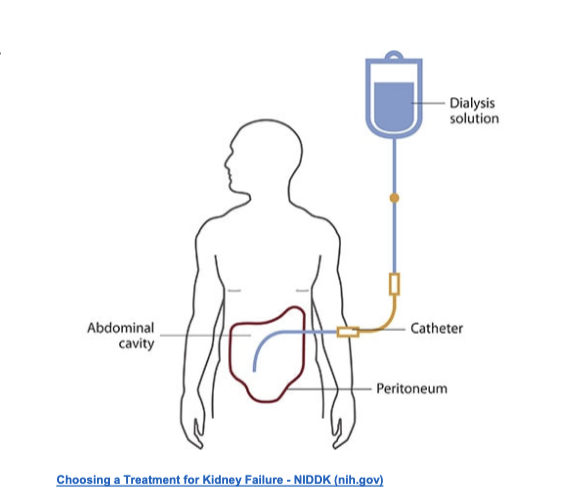
type of dialysis that uses the peritoneum in a person’s abdomen as the membrane through which fluid and dissolve substances are exchanged with the blood
to remove excess fluid, correct electrolyte problems, and remove toxins in those with kidney failure
renal replacement therapy: peritoneal dialysis pt 2
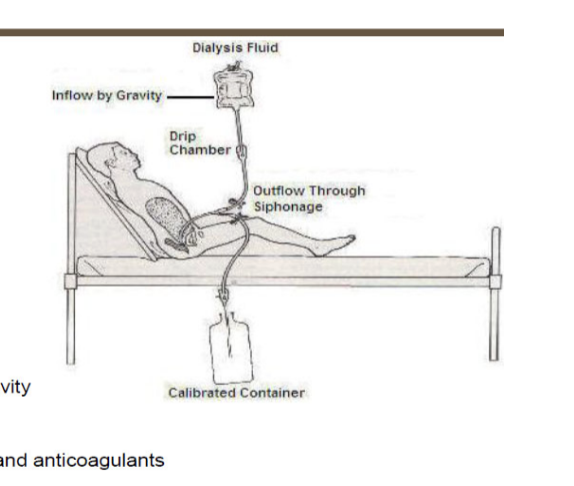
peritoneal dialysis
solution infused into the peritoneal cavity
peritoneal membrane acts as dialyzer
for patients who are hemodynamically stable
peritoneal physiology
contains ~10 mL liquid
can expand to hold several liters
surface area of 1-2 m2
allows passage of larger MW substances
catheters = used to gain access to peritoneal cavity
dialysate
high dextrose solution containing various solutes and anticoagulants
renal replacement therapy: peritoneal dialysis pt 3
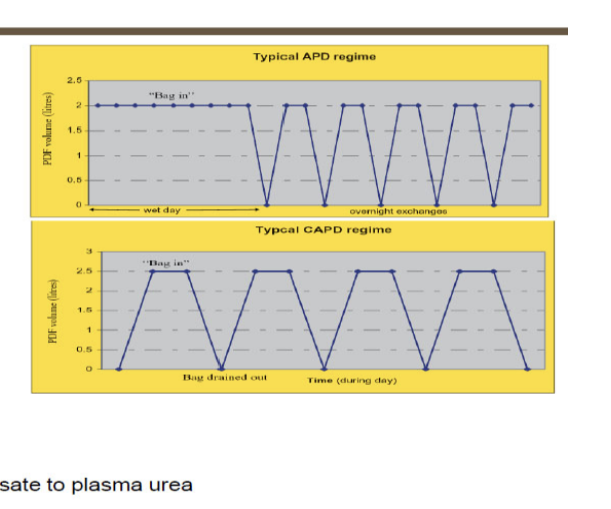
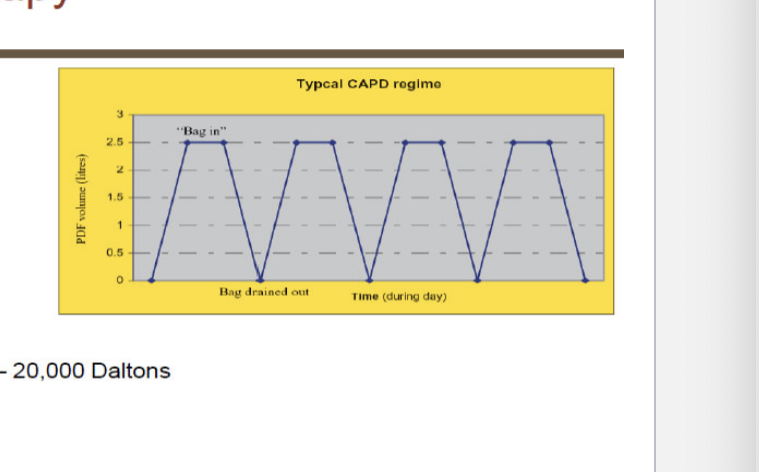
types of peritoneal dialysis
continuous cyclic peritoneal dialysis
cycler at night
day dwell
continuous ambulatory peritoneal dialysis
3 daily exchanges
1 long bedtime dwell
measures of adequacy
Kt/V where Kt = D/P * volume drained
D/P = dialysate to plasma urea concentration
should be ~2.0 per week
renal replacement therapy: peritoneal dialysis pt 4
peritoneal dialysis prescription
# of exchanges (CAPD)
volume
concentration of solutes
properties of dialyzable drug
Vd <1L/kg
protein binding <96%
can better clear large molecules up to 15,000 - 20,000 Dab
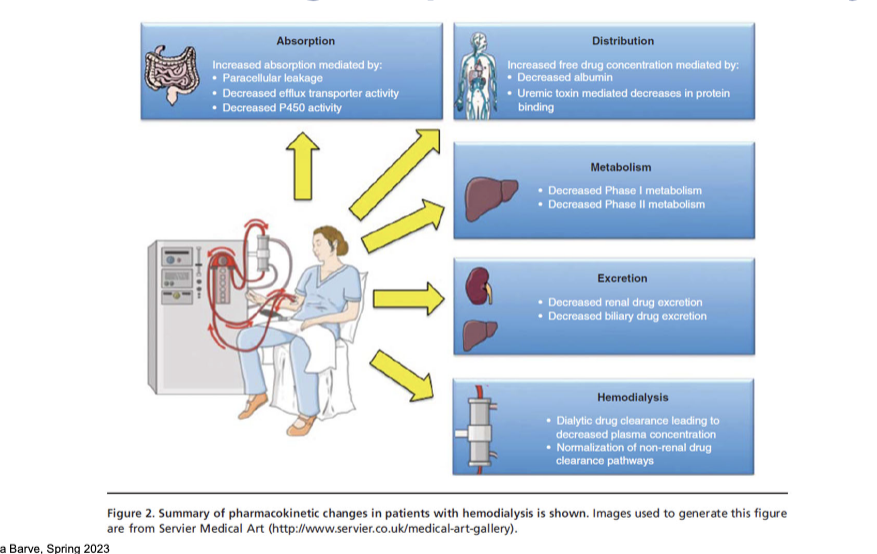
PK changes in patients with hemodialysis
absorption
increased absorption mediated by:
paracellular leakage → damaged gut barrier allows more drug to pass through
decreased efflux transporter activity → less drug is pumped out, more stays
decreased P450 enzyme → less 1st pass metabolism
distribution
increased free drug concentration mediated by:
decreased albumin → fewer binding sites for drugs
uremic toxin mediated decreases in protein binding → displaces drugs from protein
metabolism
decreased phase I metabolism
decreased phase II metabolism
excretion
decreased renal drug excretion
decreased biliary drug excretion
hemodialysis
dialytic drug clearance leads to decreased plasma concentration
normalization of non-renal drug clearance pathways
CKD: effects on the PK of drugs
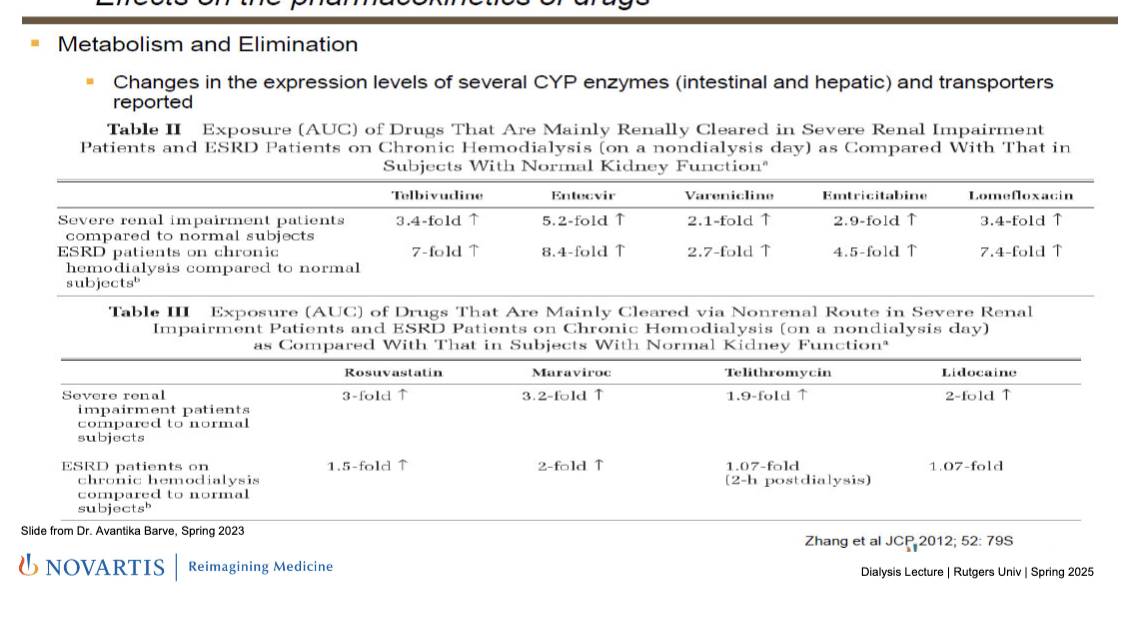
metabolism and elimination
changes in the expression levels of several CYP enzymes (intestinal and hepatic) and transporters reported
drugs that are renally cleared: impaired kidneys → less elimination b/c of the CYP enzymes that are expressed less → increased AUC
telbivudine
entecavir
varenicline
emtricatibine
lomefloxacin
clears only intermittently during HD so drug concentrations build up in between
drugs that are NOT cleared via renal (mainly hepatic); renal impairment still increases AUC due to reduced liver metabolism and uremic toxins affecting enzyme/transporter function but dialysis helps lower AUC
rosuvastatin
muraviroc
telithromycin
lidocaine
HD helps by removing some toxins which may suppress some liver enzymes so the amount by which the drug increases in circulation is less
evaluating the influence of dialytic therapies on the PK of a drug
primary questions
whether the drug dosage should be adjusted b/c of dialysis
if so, by how much
timing of drug admin relative to dialysis
intermittent hemodialysis (IHD)
most common dialysis method used in ESRD pts in U.S.
study to include both on and off dialysis periods
important to record the blood flow (QB), dialysate flow (QD) and the make and model of the dialyzer used in study to interpret study results and extrapolate to other dialysis conditions
continuous renal replacement therapy (CRRT)
for critical care medications likely to be used in patients on CRRT, findings from IHD studies might NOT be sufficient to derive dosing recommendations for pts using this modality
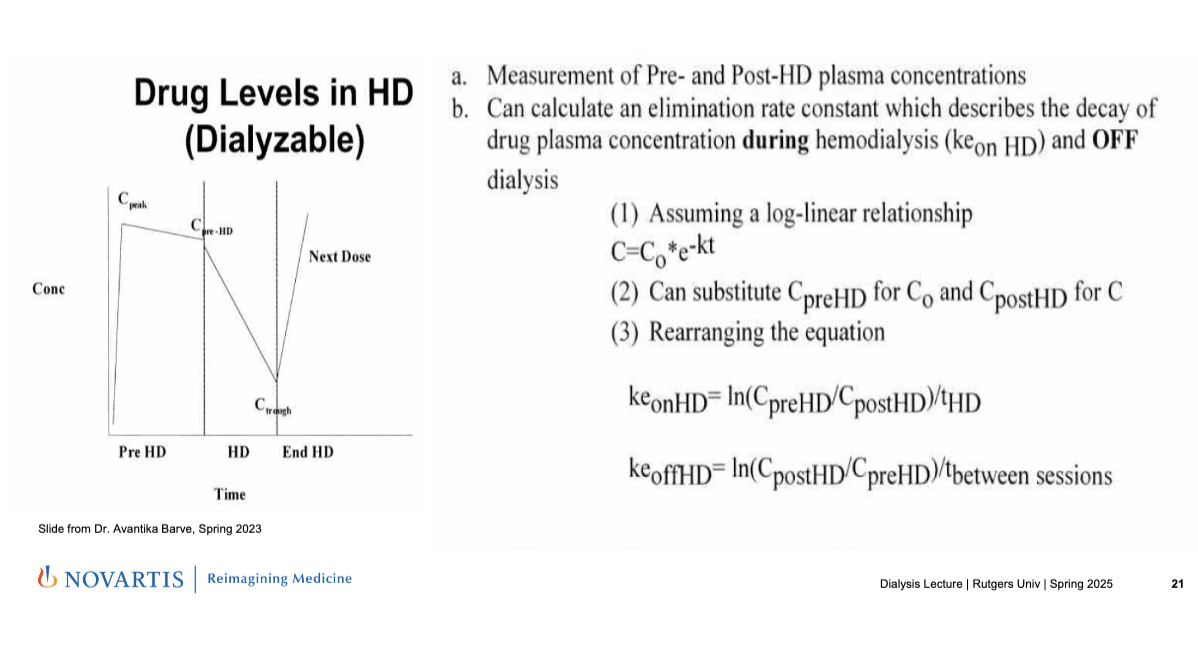
monitoring drug levels in hemodialysis
measurement of pre- and post-HD plasma concentrations
can calculate an eliminate rate constant which describes the decay of drug plasma concentration during hemodialysis (keon HD) and OFF dialysis
assuming a log-linear relationship: C = Co *e-kt
C0 = CpreHD
C = CpostHD
rearranging equation
keonHD = ln(CpreHD/CpostHD)/tHD
keoffHD = ln(CpostHD/CpreHD/tbetween sessions
graph
Ctrough = lowest level before the next dose is given
quantification of drug loss during hemodialysis
calculate fraction of drug loss during hemodialysis (fL) (fraction of drug in plasma that is removed by dialzyer)
fL = 1-e-kt (use kd)
calculate fraction of total elimination occurring during HD (fD) (of all the ways the body eliminates drug, what fraction of this total elimination is due to HD)
fD = 1-(t1/2 on HD/t1/2 off HD)
fraction of drug initially in the body that is eliminated by HD (fel) (combo of how much drug is in plasma and how much total elim is done by HD; overall impact of HD on drug elimination; how much of the total drug in the body that is actually cleared during HD session)
fel = fD * fL
may be significant if >30%