fermentation
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
what limiting reagents do we have to consider outside of intmd in glycolysis
NAD+ → for step 6, [NAD+] is much lower than the [glucose] so its the LR
if NADH inc then NAD+ dec (inverse relationship bc NADH is just NAD with H)
if lot of glucose metabolized and cell is in low pO2 environ (meaning no cellular respiration) then it runs out of NAD+ and uses fermentation to regen it
what does fermentation produce
lactate in humans and ethanol + CO2 in yeast
fermentation
allows glycolysis to continue without O2 - w/ O2, glycolysis goes straight into cellular respiration and regens NAD+ but w/o, NAD+ runs out and stops glycolysis
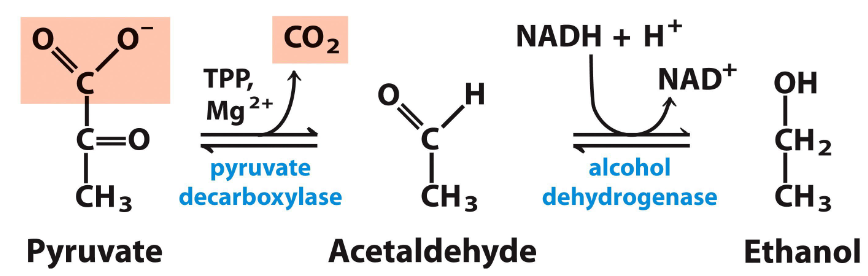
yeast and alcohol fermentation
goes from pyruvate to acetaldehyde to ethanol by pyruvate decarboxylase (releases CO2) and alcohol dehydrogenase (regen NAD+)
ethanol then diffuses out of the cell
why does dough rise and beer carbonated
yeast mostly use glycolysis to power themselves and fermentation to regen NAD+, which releases CO2 and ethanol
why does beer have low [alcohol]
bc the yeast strains used to brew it can only tolerate so much [ethanol] and [acetaldehyde] - both are toxic to yeast
TPP
thiamine phyrophsphate
cofactor involved with decarboxylase and releasing CO2
made from vitamin B2 and is integrated into the e (like heme group for hemoglobin)
important part of TPP
thazolium ring deprots to make a v reactive carbanion and acts as an e- acceptor to facilitate rxns
every other part of the molec binds it to the e and stabilizes
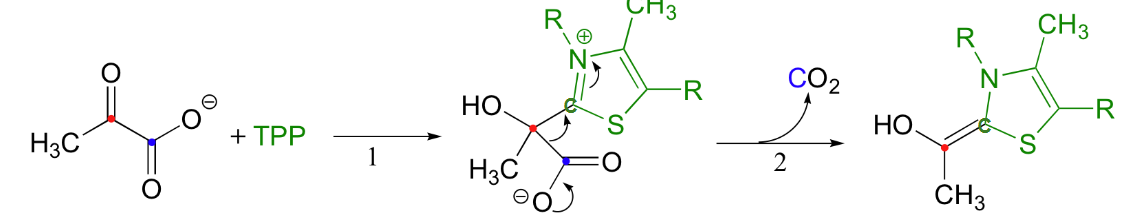
mechanism of TPP
binds to pyruvate to make tetrahedral intmd, the -O: pushes e- down to make 2x bond and release CO2, then forms acetaldehyde
first step of fermentation (acetaldehyde reduced to EtOH and NADH to NAD+)
humans and first step of fermentation
in the liver, alcohol dehydrogenase cat reverse rxn and oxidizes EtOH to acetaldehyde, consumes NAD+
lactate fermentation
humans - active muscle tissue uses NAD+ faster than it makes it (glycolysis > CR) so oxidize NADH to NAD+ by reducing pyruvate to l-lactate
allows glycolysis to cont under anerobic conditions so we don’t pass out whenever we exercise
pyruvate to lactate
uses lactase dehydrogenase
v favorable, dG(`0)= -25
regens NAD+
inc [lactate (acid)] = dec pH, contr to hemog T state stabilization in muscle tissues
![<p>uses lactase dehydrogenase<br>v favorable, dG(`0)= -25<br>regens NAD+<br>inc [lactate (acid)] = dec pH, contr to hemog T state stabilization in muscle tissues</p>](https://knowt-user-attachments.s3.amazonaws.com/ee359c73-8569-4194-b6cf-9171ec9beb4c.png)
gluconeogenesis purpose
making glucose instead of breaking it down
done bc we have tissues that only want glucose as energy source and need constant input (nervous sys, brain, red blood cells (literally just sac of hemog w/o mito so no CR), embryonic tissues)
we can’t ensure constant supply of glucose so need to make it
animals make glucose from what
lactate, pyruvate, some aa, glycerol
plants can use CO2
where does gluconeogenesis (gng) occur
partially in the cyotoplasm (where all the reversible steps happen) and mito (where gng specific e are) to prevent a futile cycle and bc pyruvate immediately shuttled into there when made
how regulate gng vs glycolysis
gng and glycolysis reg at the steps where they differ (1, 3, 10) bc reg at reversible (non-1,3,10 steps) affects both and that’s not super helpful
diff btwn gng and glycolysis pathway wise and why
can’t have same pathway for both - they need an overall dG=- and that’s not possible if they use the same pathway
differ at irreversible steps : 1, 3, 10 by going backwards at those steps is really unfavorable
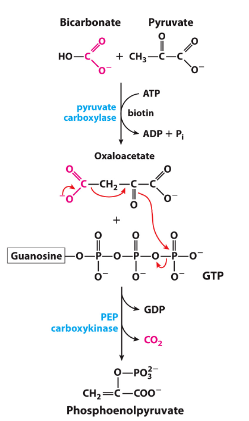
bypassing steps 1, 3, 10
step 10: requires gtp (~same energy as atp) so used up all energy made in glycolysis
pyruvate + bicarbonate →pyruvate carboxylate→ oaa + gtp →pep carboxykinase→ pep + co2 + gdp
step 1:
glucose 6-phosphate →glucose-6phosphatase→ glucose
step 3:
fructose 1,6-bp → fructose 1,6-biphosphatase-1→ fructose 6P
when is gng used
sleeping, fasting, starvation, exercise
spatial reg of gng
1. 4 gng enzymes are expr only in the liver (sequestered) while glycolysis can happen in every cell - glucose from liver then transported to other places
2. alt step 10 of gng uses pyruvate carbylase, which is only in the mito bc pyruvate is immediately shoved in there
goal of reg gng
prevent futile cycle where you make glucose and then it immediately gets used and converted into pep, then pyruvate, then converted back into pep and glucose
on a molec level, how is a futile gng cycle prevented
spatial separation of hexokinases that kick off glycolysis:
hexokinase i, ii, iii in the muscle tissues have a high affinity for glucose and are pretty much always running at Vmax (bc muscles always need ATP)
hexokinase iv (glucokinase) is in the liver and has a lower affinity, isn’t always running at Vmax - allows for inc or dec activity in response of blood sugar levels. higer Km allows pdced glucose to escape the liver and get to other tissues (hk i, ii, or iii would just immediately capture it)
hexokinase i and ii reg
glucose 6p acts as am - if the rest of the sys can’t keep up with hexokinases, then the pdct binds and dec activity
hexokinase iv reg
fructose 6p acts as reg when inc in C (means sys can’t keep up) reg proteins bind to hexokinase iv and sequesters it in the nucleus to prevent glycolysis from getting started
when [glucose] inc, its released into cytoplasm to start glycolysis
pentose phosphate pathway
generates pentoses and reducing equivalents in the form of NADPH
takes glucose 6p and converts it into ribulose 5p which can be turned back into glucose 6p or into ribose 5p
generates nadph and uses nadp+
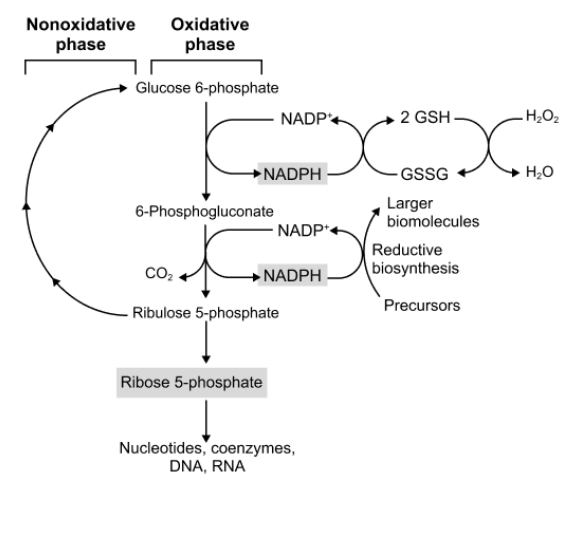
ribose 5p
used to make nucleotides, coenzymes, DNA, and RNA
nadph as reg for pentose pathway
inc Concentration inhibits the first step and allows for more g6p to undergo glycolysis
how does the pentose phosphate pathway connect to CR and glycolysis
under oxidative stress, CR can sometimes make H2O2 instead of H2O so pentose phosphate pathway used to reduce it