Synthesis Ketones and Aldehydes
1/143
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
144 Terms
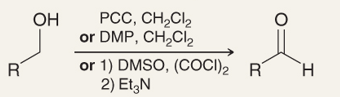
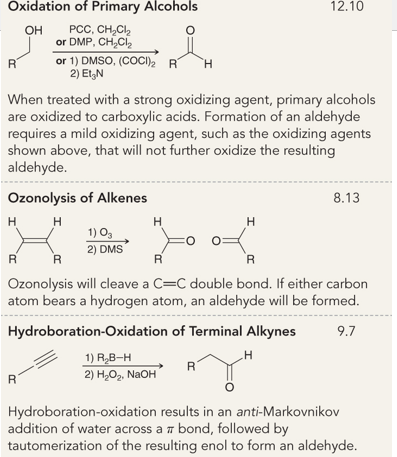
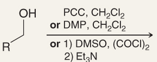
What is the name of this reaction
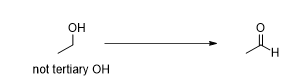
the oxidation of primary alcohols
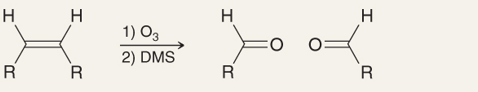
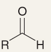
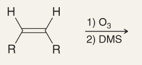
What is the name of this reaction
Ozonolysis of Alkenes
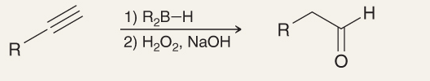
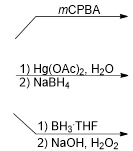
What is the name of this reaction:
Hydroboration- Oxidation of Terminal Alkynes
What do all of these reaction have in common
They are the ways that you can make aldehydes
What will be the product of this reaction
What would be the product of this reaction
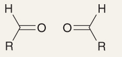

What will be the product of this reaction?
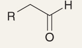
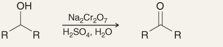
What is the name of this reaction:
Oxidation of Secondary Alcohols
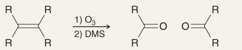
What is the name of this reaction
Ozonolysis of Alkenes
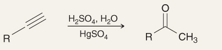
What is the name fo this reaction:
Acid Catalyzed Hydration of Terminal Alkynes
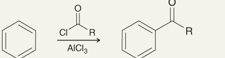
What is the name of this reaction
Friedel Crafts Acylation
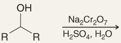
What is the product of this reaction
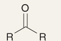

What is the product of this reaction
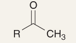
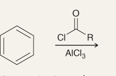
What is the product of this reaction
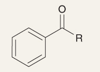
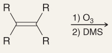
What is the product of this reaction
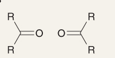
Which reaction is being shown
Hydration
What is the main reagent in hydration
OH2
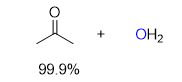
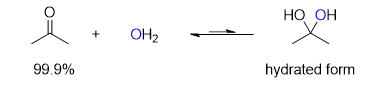
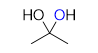
what should be the product of a reaction like this:
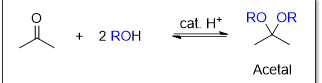
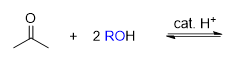
What type of reaction is this
Acetal formation
what is the overall product from this reaction
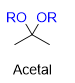
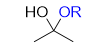
What is this?
hemiacetal- youre halfway through the mechanism
When is a catalyst consumed and regenerated
during protonation and deprotonation
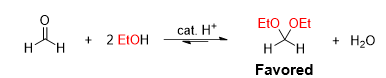
What type of reaction is this
Acetal formation
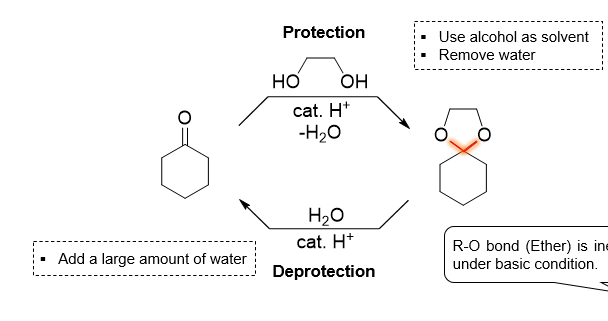
What is being shown here
Acetal being a protecting group
In terms of acetal as a protecting group, what does thsi specific reaction do
attach the protecting group
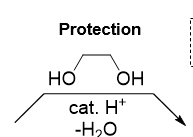
What do you add and remove in placing a protecting group
add cat H ( using alcohol as a solvent) and remove H2O
What do you add and remove in removing a protecting group
add H2O and remove cat. H
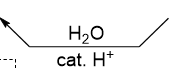
in terms of using acetal as a protecting group , what does this do
deprotection
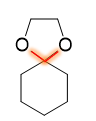
what causes this product
when an acetal becomes a protecting group
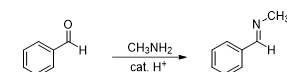
What kind of reaction is this
Imine formation
What reagent is used in imine formation
CH3NH2
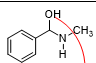
what is this and what reaction can it be found in
carbinolamine
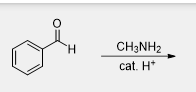
What is the product of this reaction
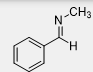
Many RNH2 reacts with aldehydes and ketones including compounds in which R is not an alkyl group, what is another alternative used as a reagent
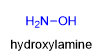
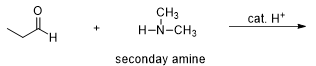
What will be the resulting product of
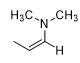
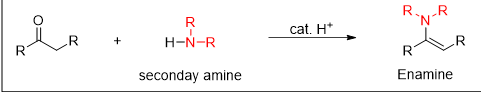
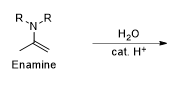
What is the reaction being shown
Enamine formation
what is the difference between the last steps of their reactions of enamine and imine?
imine uses deprotonation compared to enamine which uses elimination
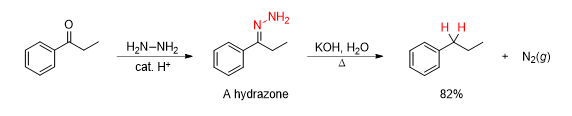
Which reaction is being shown
Wolff Kishner Reduction
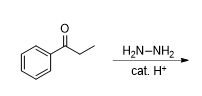
What does this reaction produce
halfway through the reaction of a wolf kishner reaction
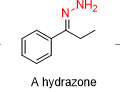
What is the reagent in a wolff kishner reduction in the beginning before the KOH,H2O/heat
H2N-NH2/cat H
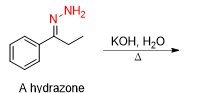
What will be the product from this
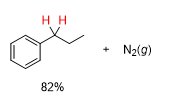
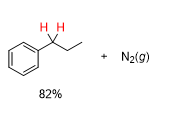
whats the reagent from a hydrazone to in a Wolff Kishner Reduction
KOH, H2O/heat
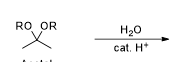
what is the product of
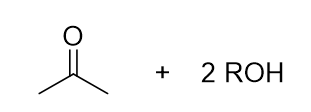
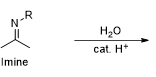
what is the product of
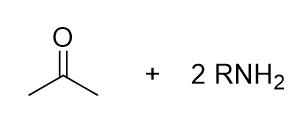
what is the product of
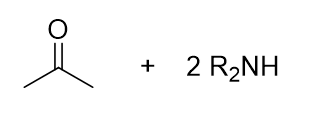
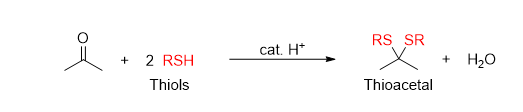
What reaction is being shown
Thioacetal Formation
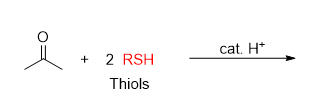
What is the product from this reaction
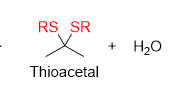
How do you remove thioacetal
desulfurization
Here a ketone is protected by a placed protecting group .. what will the product look like
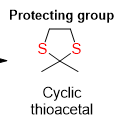
To further get rid of the thioacetal from the protecting group there can be 2 reagents applied.. what are they
H2 Rainey Ni
NgCl2
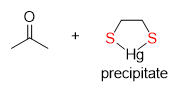
What is the product of a protecting group that undergoes a reaction with the reagent HgCl2
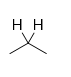
What is the product of a protecting group that undergoes a reaction with the reagent H2 Raney Ni
What are the reducing agents of aldehydes and ketones
H2/Pd
NaBH4
LiAlH4 (LAH)
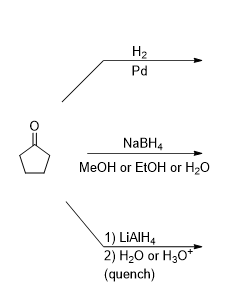
What product do these all lead to
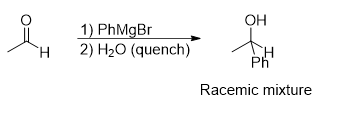
What reaction is being shown
Grignard Reagent
What is the reagent used in grignard reagents
PhMgBr
H2O
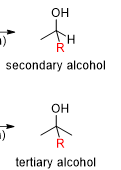
Grignard reagents have different products for aldehydes and ketones what are the products for each individual one
aldehydes make a secondary alcohol
ketone makes a tertiary alcohol
How do you make a Grignard Reagent
alkyl halide ( R-X)
1. Mg, ether / 2. H2O (quench)
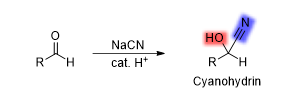
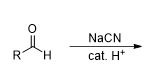
What is the reaction being shown
Cyanohydrin Formation
What is the reagent in Cyanohydrin Formation
NaCN/cat. H^+
What is the product from this reaction
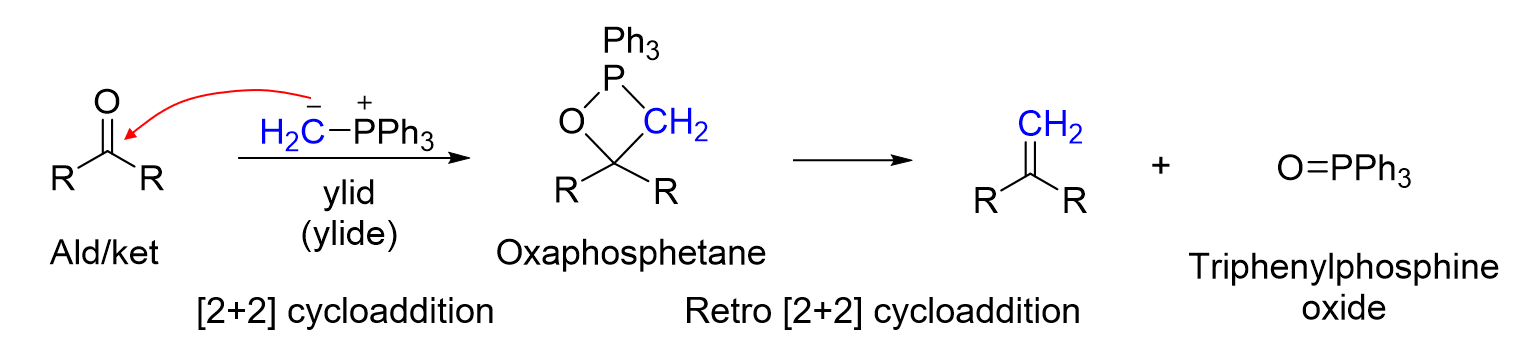
What reaction has is being shown
Wittig Reaction
What is the product from this reaction
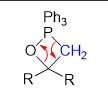
What is the overall product from this
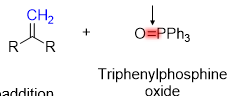
How do you make a Ylid reagent
alkyl halide
1)PPh2 (Sn2) / 2.) n-BuLi ( Elimination )
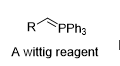
How do you make this?

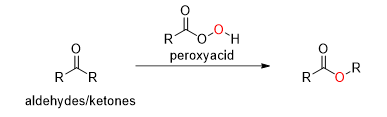
What is this reaction
Baeyer Villiger Oxidation
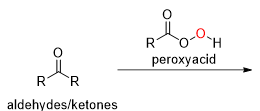
What is the product of this

When going from an alkyne to an alkene what reagent would you use for that
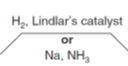
What reagent would you use to go from an alkene to an alkane
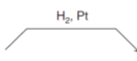
How do you go from an alkane to an alkene
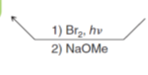
What reagents do you use to go from an alkene to an alkyne

What reagents do you use to go from a secondary alcohol to a ketone
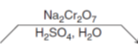
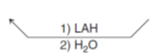
What reagents do you use to go from a ketone to a secondary alcohol
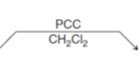
What reagents turn a primary alcohol into aldehyde
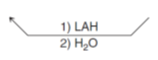
What reagent turns a aldehyde back into a primary alcohol
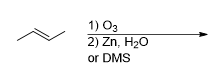
What is the product for this reaction
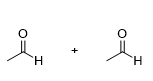
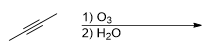
What is the product for this reaction
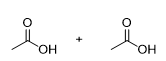
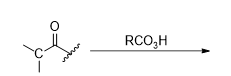
What is the product for this reaction
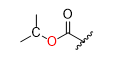
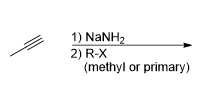
What is the product for this reaction
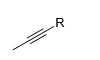
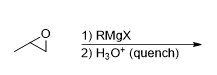
What is the product for this reaction
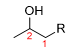
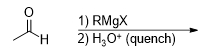
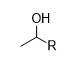
What is the product for this reaction
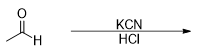
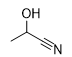
What is the product for this reaction
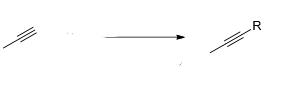
What reagent would be used to
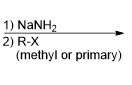

What reagent would be used to
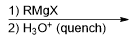

What reagent would be used to
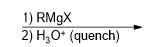

What reagent would be used to
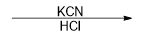
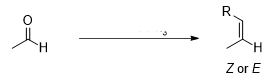
What reagent would be used to
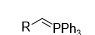

What reagent would be used to
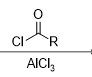

What reagent would be used to
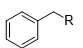
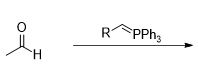
What is the product of this reaction
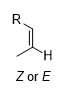
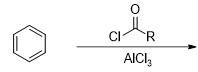
What is the product of this reaction
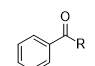
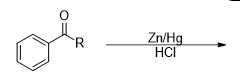
What is the product of this reaction
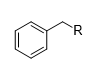
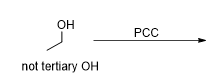
What is the product of this reaction
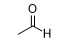
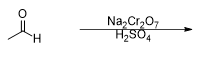
What is the product of this reaction
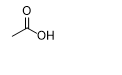
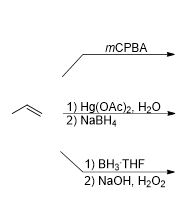
What is the product of this reaction
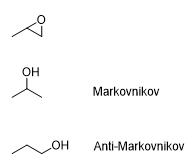
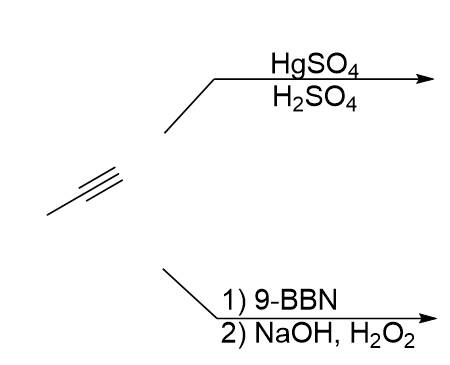
What is the product of this reaction
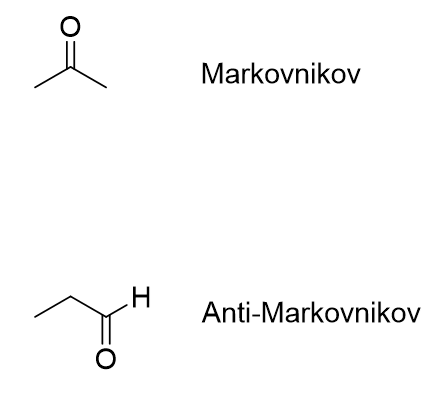
What is the reagent that results in this
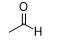

What is the reagent that results in this
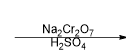
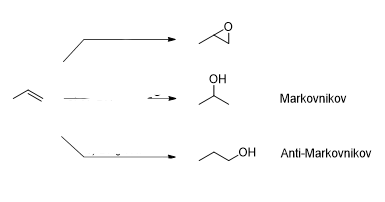
What is the reagent that results in this