Topic 3 - Bonding: Lewis Dot Structures
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Lewis Dot Structures – Overview
Purpose: A systematic method to determine and represent molecular structure from molecular formulas.
Representation: Uses dots for electrons and dashes for covalent bonds.
Model Limitation: Represents electrons as particles, which ignores wave-particle duality (QM). Useful for predicting structure but not fully accurate—experimental data is needed for the real structure.
*Best applies to covalent bonds rather than ionic bonds
Covalent Bond
Electron sharing between atoms.
Usually between nonmetals.
Forms molecules.
Bonds can be single, double, or triple.
Example: H₂O, CO₂.
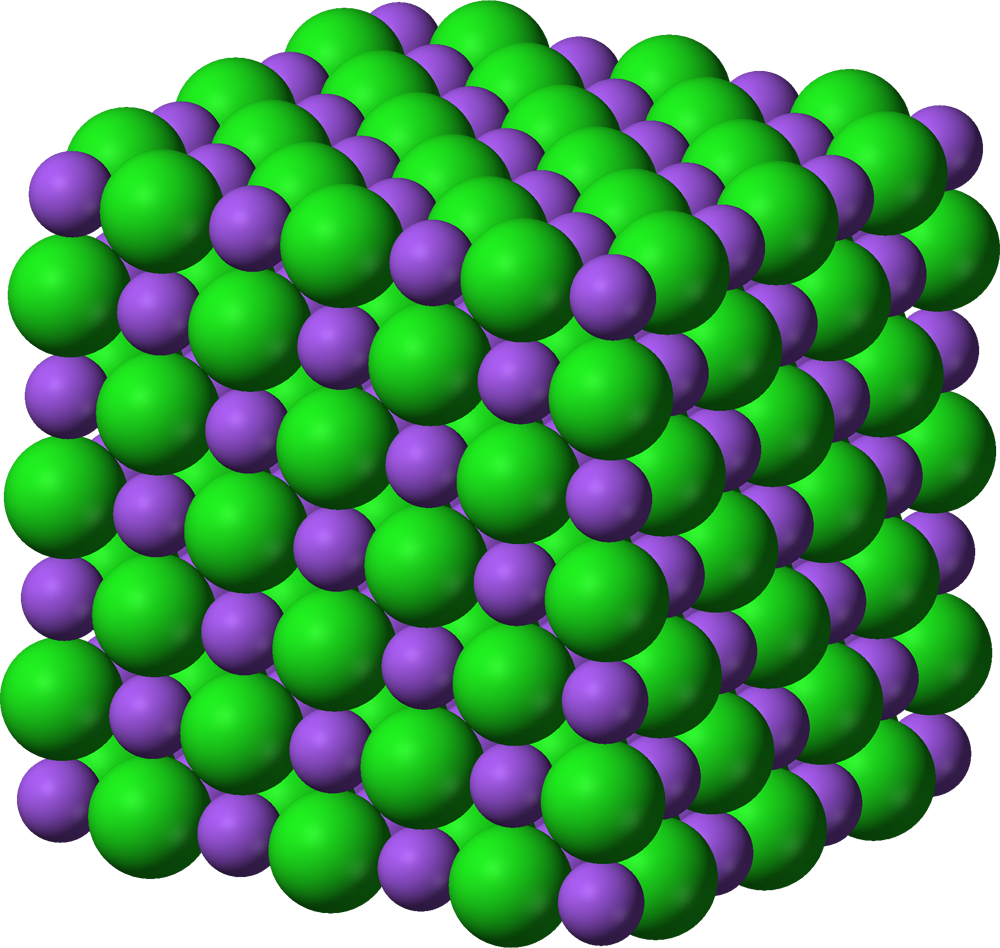
Ionic Bond
Electron transfer from one atom to another → ions.
Usually between metal + nonmetal.
Forms crystal lattice.
Bond due to electrostatic attraction between oppositely charged ions.
Example: NaCl, MgO.
Lewis Dot Structures - Approaches: Octet/Duet Rule Approximation
Valence electrons (electrons involved in bonding) are determined by periodic table group number (last digit). *excluding actinides, lanthanides, and transition metals for now....
Representation
Bonding electrons: Each dash represents 2 electrons (in total) shared between atoms.
Lone pairs: Dots arranged in pairs around atoms - each dot represents an electron not involved in bonding.
Goal: Each atom reaches an octet (or duet for H/Li).
*Core electrons not shown, only valence.
Lewis Dot Structures - Approaches: Octet/Duet Rule Approximation Steps
1. Count total valence electrons (group number) for all atoms in the molecule.
When there's an ion; Add electrons to the total electrons if it's a negative charge (-), subtract electrons from the total electrons if it's a positive charge (+).
2. Lay out atoms involved and form single bonds (one pair of electrons) between bound atoms.
H max 1 bond.
Central atom = least electronegative atom (closer to the bottom-left hand of the periodic table) - except for H. The atom that appears only once is usually the central atom
3. Distribute remaining valence electrons (from the total) as lone pairs (outer atoms first) to satisfy the octet/duet rule.
4. Check octet/duet for all atoms.
Add multiple bonds if needed (use lone pairs from more electronegative atoms first - closer to the right-hand side of the periodic table).
*For charged species (ions), enclose the diagram in brackets with charge as superscript.
Core Electrons
Non-valence electrons; not involved in bonding.
Valence Shell
Outermost shell containing valence electrons (involved in bonding).
Octet Rule
Atoms aim for 8 valence electrons (stable configuration of noble gases).
Duet Rule
For H, Li, (and He) they aim for 2 valence electrons for stability (stable configuration of the nearest noble gas - He)
*Though, Lithium itself doesn’t follow the duet rule — but when it loses one electron, the resulting Li⁺ ion ends up with a full first shell (duet), which is stable.
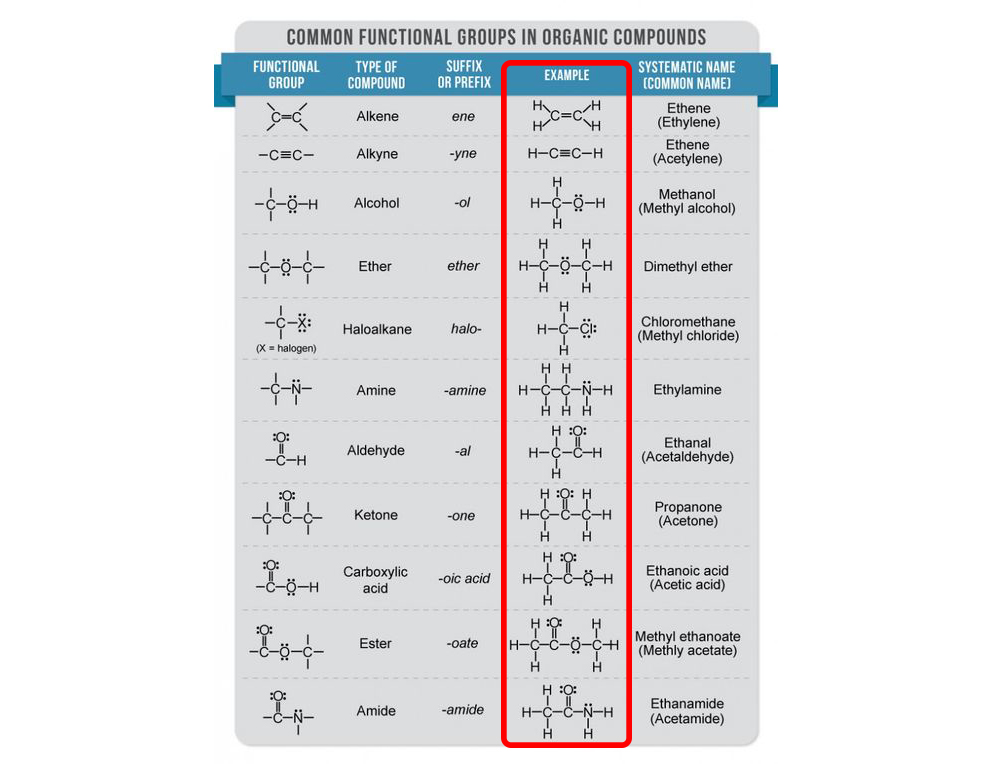
Organic Lewis Structures
Organic = Carbon backbone + hydrogen (hydrocarbons); in its simplest form are alkanes (singly bonded carbons with hydrogens) but the complexity increases with functional groups.
Common functional groups to recognize: alkanes, alkenes, alkynes, alcohols, carboxylic acids, esters, ketones, aldehydes, ethers, amines, amides.
Formula conventions:
Atoms listed in bonding order from left to right in the chain.
With the central chain as the least electronegative atom, except for hydrogen.
Most organic species obey the octet/duet rule.
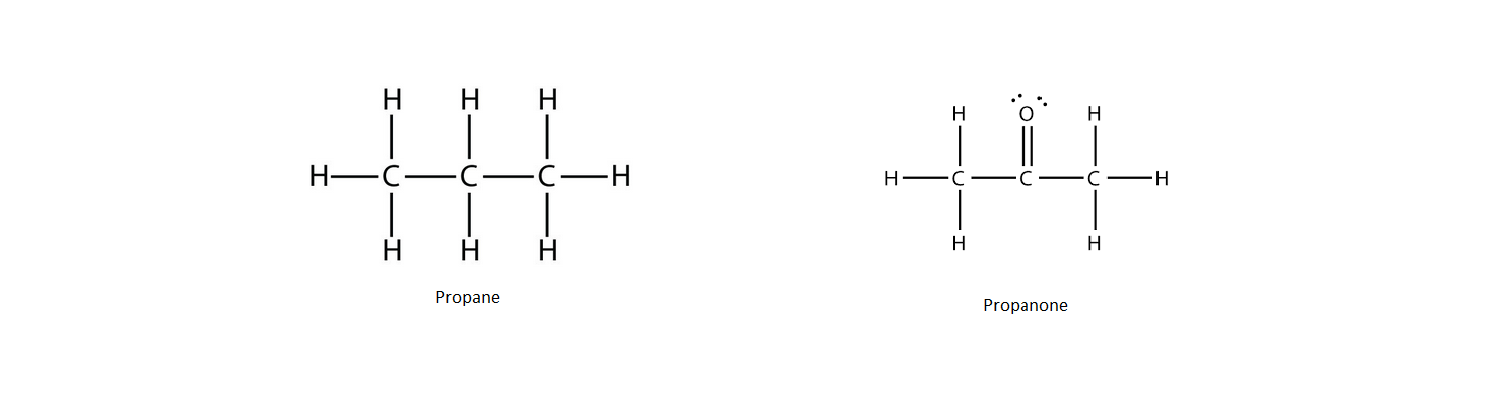
Examples:
Propane (CH₃CH₂CH₃)
Propanone (CH₃COCH₃)
Hydrocarbon
Organic molecules made of only hydrogen and carbon atoms
Inorganic Lewis Structures
Inorganic = Non-organic species, no C–H bonds.
N₂, CO2, and many metal-containing compounds.
Examples obeying octet/duet: NH₃, H₂O.
Exceptions: BeCl₂, BCl₃ (incomplete octets) → use formal charge approach.
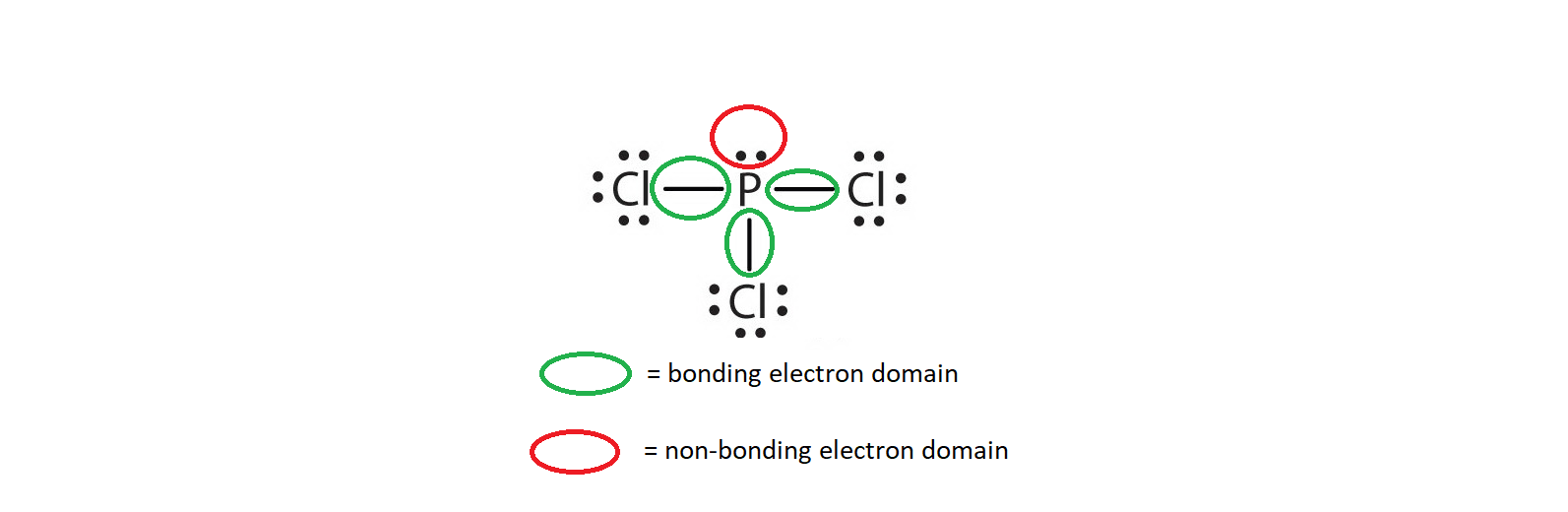
Electron Domains
Bonding Electron Domain: Electrons in bonds (shared).
Non-Bonding Electron Domain: Electrons in lone pairs.
Electron Domains: Number of lone pairs + bonding locations around an atom.
Lewis Dot Structures - Approaches: Formal Charge
Why: Octet rule/duet rule alone may not give most stable structure.
Appropriate for...
Molecules that don't follow the octet rule
For atoms that can exceed the octet rule, use this approach to decode if it's worth giving an atom more than 8 electrons (have access to d-orbitals).
When unsure of where to place extra valence electrons
When there are multiple valid Lewis structures to choose from
What: Comparing the amount of valence electrons in a free atom (not bonded) vs. the amount of valence electrons actually around the atom in the molecule.
Lewis Dot Structures - Approaches: Formal Charge Steps
Formula: Formal Charge = (# of the periodic valence electrons (group number)) - (# electrons in lone pairs + 1/2 # electrons bonded)
Apply to each atom in a molecule
Formal charges close to zero have the most stable Lewis structure
A negative (-) formal charge indicates too many electrons attributed
A positive (+) formal charge indicates too little electrons attributed
To fix, take a lone pair from a negative formal charge and turn it into a bond
Exceptions to the Octet Rule - Incomplete Octet
Atoms that are stable with fewer than 8 valence electrons:
Hydrogen – stable with 2 electrons (e.g., H₂, HCl)
Helium – stable with 2 electrons (noble gas)
Lithium – stable with 2 electrons (e.g., LiH)
Beryllium – stable with 4 electrons (e.g., BeCl₂)
Boron – stable with 6 electrons (e.g., BF₃, BCl₃)
Aluminium – sometimes stable with 6 electrons (e.g., AlCl₃)
Exceptions to the Octet Rule - Expanded Octet
Atoms in the third period or beyond (n ≥ 3) can have more than 8 electrons because they can use d-orbitals:
Phosphorus – up to 10 e⁻ (e.g., PCl₅)
Sulfur – up to 12 e⁻ (e.g., SF₆, SO₄²⁻)
Selenium - up to 12 e⁻ (e.g., SeF₆, SeCl₄)
Chlorine – up to 14 e⁻ (e.g., ClF₃, ClF₅, ClO₄⁻)
Xenon – up to 14 e⁻ (e.g., XeF₂, XeF₄, XeO₃)
Iodine – up to 14 e⁻ (e.g., IF₇, ICl₄⁻)
Bromine - up to 14 e⁻ (e.g., BrF₃, BrF₅)