Dementia
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Symptoms from a case studies
grammar uunaffected, semantic and episodic impaired, filler words,
What is dementia?
Dementia is an umbrella term used to describe chronic widespread cognitive impairment, associated with significant changes to functional abilities. The impairments may have a number of causes, including Alzheimer’s disease, and they are progressive and largely irreversible.
“Dementia” was a common term for cognitive decline in aging. • “dementia praecox” was used for dementia earlier in life (e.g. before 65), it was often used interchangeably with schizophrenia. • Later, use of “Alzheimer’s disease” to distinguish from dementia, and age was dropped as a criterion for diagnosis. • Scope of dementia/Alzheimer’s disease determined in the 1950s and 1960s.
What is dementia?
An estimated 55 million people worldwide have dementia (World Health Organization, 2022), and about 900,000 people in the UK (Alzheimer’s Society, 2021). • Prevalence is rising. • Among the risk factors: genes, vascular factors (diabetes, hypertension), lifestyle (e.g. alcohol and smoking), education and social engagement. • Not curable (but treatable). • Immense costs to societies (more than £15 billion in the UK), plus hidden costs.
Dementia overview
Alzheimer’s disease (AD; 50 – 75%) • (Typical) amnestic AD • Logopenic variant primary progressive aphasia • Posterior cortical atrophy, Semantic and episodic memory, visual impairment
Lewy Body Dementia • Dementia with Lewy Bodies • Parkinson’s disease, motor difficulties
Frontotemporal dementia • Behavioural variant • Semantic primary progressive aphasia • Non-fluent variant primary progressive aphasia chnages in diets, habits, behaviour
Vascular dementia • Subcortical vascular encephalopathy • Strategic infarct dementia • Multi infarct dementia
Very small lesions over time cumulative of strokes
Current care pathway
Possible symptoms often observed by family and friends, or experienced by the individual. 2. GP assesses the individual to exclude other causes. 3. Referral to a neurologist / Memory clinic. • Exclusion of other causes. • Interview, cognitive screening (e.g. MMSE, MoCA, ACE) • Can be assisted by neuroimaging (CT, MRI, PET) • Outcomes: No dementia (“worried well”), mild cognitive impairment (MCI), dementia • MCI can be an early stage of dementia, or an unexplained impairment. Follow-up appointments are recommended.
The brain over the lifespan
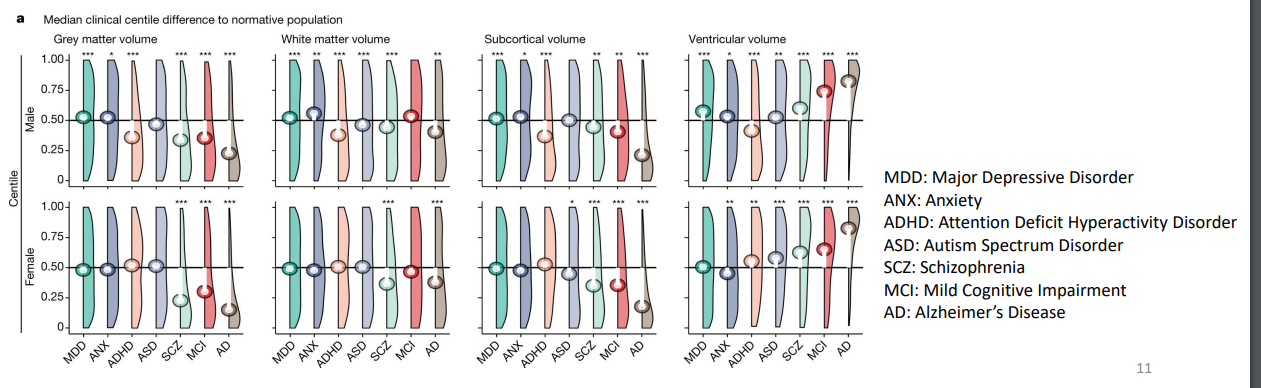
Bethlehem et al., 2022 • Based on more than 220,000 anatomical MRI scans. • Grey matter volume peaks in childhood(5.9 years), white matter volume in adulthood (28.7 years).
Different clinical diagnoses associated with differences in grey and white matter volume.
Changes in the brain
In Alzheimer’s, amyloid plaques “stick” to neurons and disrupt communication between neurons, while aggregation of tau-proteins in the cell disrupt metabolism and result in neurofibrillary tangles.
Neurodegeneration
Typical ageing (Bakkour et al., 2013): • Whole cortex MRI on 142 young controls, 87 older controls, 28 mild AD patients, and 35 older controls who were likely amyloid-negative according to PET. • Age effect mostly in sensorimotor and visual cortices. • Some overlap with AD in frontoparietal and temporal regions. • However, in the temporal lobe AD atrophy is substantially more severe.
• Overview of brain atrophy related to Alzheimers: Pini et al. (2016)
One of the core biomarkers: hippocampal atrophy • Detected in mild AD dementia and in MCI • Up to 40% volume reduction. • Hippocampus crucial for: spatial orientation (e.g. place cells), temporal context, episodic memory (especially recent memories and memory formation)
Neurodegeneration
Atrophy tends to start in the medial temporal lobe and posterior cingulate cortex (see picture). • Temporal lobe associated with lexical-semantic processing. • Cingulate cortex part of the limbic system – associated with emotion processing and learning. • Posterior cingulate cortex associated with access to episodic memory. • It extends to the rest of the cortex: temporal – parietal – frontal trajectory. • Motor and visual areas mostly spared until later stages.
Pini et al. (2016). A = grey matter developing AD vs. controls; B = mild vs. developing AD; C = moderate vs. mild AD
Atrophy to a range of subcortical areas, many associated with memory and/or learning, e.g. amygdala, basal ganglia. • White matter lesions and connectivity changes. • However, much variation, including sub-types (posterial cortical atrophy; logopenic variant primary progressive aphasia.
Language in Alzheimer‘s disease
Lexical retrieval most recognized. • Effects on intonation, pauses, speech rate. • Semantic impairment greater than phonological and perceptual (see last week). • Possible lexical effects (Cuetos et al., 2012).: • Frequency (more common = easier) • Age of acquisition (earlier = easier) • Imageability (concrete = easier) • Phonological complexity • Visual complexity (in picture naming) • Effects uneven across the literature.
Single case studies: Ronald Reagan and Iris Murdoch
RONALDHad dementia , the number of unique words decreased and the filler wordds increased throughut the years.
IRIS
British writer, last novel heavily criticized as language less sophiesticated
Language in Alzheimer‘s disease
Grammatical change much more subtle. • In mild AD, only small increase in grammatical errors (Sajjadi et al., 2012). • However, some decrease in complexity, for example canonical vs. Non-canonical forms (Bates et al., 1995). However, effect on complexity not always observed (Mueller et al., 2018).
Lewy bodies
• Spillatini et al. (1997). a) Lewy bodies (thin arrows) and Lewy neurites (thick arrows) b) A neuron with two LBs c) Extracellular LB
Most importantly, damage to the Substantia Nigra in the midbrain • Associated with motor planning, reward-oriented behaviour • Produces dopamine
Six stages according to Braak (2003)
Six stages according to Braak (2003), based on autopsy of brains of 41 people with PD, 69 people with no PD symptoms but with LB, and 58 without LB. • Stage 1: Medulla. • Stage 2: Medulla and pons. • Stage 3: Midbrain including Substantia Nigra. • Stage 4: Basal forebrain, mesocortex. • Stage 5: Neocortex (sensory areas). • Stage 6: Neocortex (premotor areas).
Parkinson’s from the gut
e.g. Liddle (2018). • Inflammation of the intestine associated with Parkinson’s disease. • Communication between gut and brain, e.g. to control intestinal functions or for immune regulation. • Microbial changes in the intestines of PD patients years before onset. • Higher levels of α-synuclein in the gut of PD patients, expressed from enteroendocrine cells.